Wavefunctions and Energy Levels - PowerPoint PPT Presentation
Title:
Wavefunctions and Energy Levels
Description:
Electron Spin ... Electrons behave like a spinning sphere, like a planet rotating on its axis. An electron has two spin states, represented by and or a and b. ... – PowerPoint PPT presentation
Number of Views:30
Avg rating:3.0/5.0
Title: Wavefunctions and Energy Levels
1
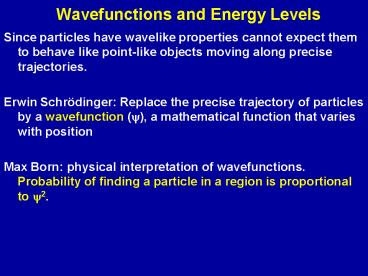
Wavefunctions and Energy Levels
- Since particles have wavelike properties cannot
expect them to behave like point-like objects
moving along precise trajectories. - Erwin Schrödinger Replace the precise trajectory
of particles by a wavefunction (y), a
mathematical function that varies with position - Max Born physical interpretation of
wavefunctions. Probability of finding a particle
in a region is proportional to y2.
2
- y2 is the probability density. To calculate the
probability that a particle is in a small region
in space multiply y2 by the volume of the region.
- Probability y2 (x,y,z) dx dy dz
3
Schrödinger Equation
- The Schrödinger equation describes the motion of
a particle of mass m moving in a region where the
potential energy is described by V(x).
(1-dimension)
Only certain wave functions are allowed for the
electron in an atom The solutions to the equation
defines the wavefunctions and energies of the
allowed states An outcome of Schrödingers
equation is that the particle can only possess
certain values of energy, i.e. energy of a
particle is quantized.
4
- In the H atom the potential that the electron
feels is the electrostatic interaction between it
and the positive nucleus - V(r ) - e2 / (4 p eo r)
- r distance between the electron and the nucleus.
- Solve the Schrödinger equation to determune the
allowed energy levels of an electron in the H
atom - Solution for allowed energy levels is
R (me e4) / (8 h3 eo2) 3.29 x 1015 Hz
5
n principle quantum number. Labels the energy
levels When n 1 gt ground state of the H atom.
Electron in its lowest energy n gt 1 excited
states energy increases as n increases E 0
when n 8 , electron has left the atom -
ionization
6
Atomic Orbitals
- Wavefunctions of electrons in atoms are called
atomic orbitals, have a dependence on position - Square of the wavefunction - probability density
of electron - The wavefunction of an electron in a hydrogen
atom is specified by three quantum numbers,
specifying energy and probability of finding an
electron. - 1) Principle quantum number, n specifies energy
of the orbitals. In a hydrogen atom, all atomic
orbitals with the same value of n have the same
energy and are said to belong to the same SHELL
of the atom.
7
- 2) Orbital angular momentum quantum number, l
- l 0, 1, 2, ., n-1
- Each value of l corresponds to a different type
of orbital with a different shape - The orbitals of a shell with principal quantum
number n fall into n groups, called SUBSHELLS
each subshell is identified by a different l
value. - l 0 s-orbitals
- l 1 p-orbitals
- l 2 d-orbitals
- l 3 f-orbitals
8
- Magnetic quantum number, ml distinguishes the
orbitals within a subshell. Determines how the
atom behaves in a magnetic field. - ml l, l -1, - l
- 2 l 1 ml values for each l
- l 1 ml 1, 0, -1
n is related to the size of the orbital, l is
related to its shape, and ml is related to its
orientation in space.
9
(No Transcript)
10
- s orbitals correspond to l 0 and ml 0
- For Hydrogen atom the ground state is n 0, l
0 and ml 0 a s orbital
Density of shading represents the probability of
finding an electron at any point. The graph shows
how probability varies with distance
11
- Wavefunctions of s orbitals of higher energy have
more complicated radial variation with nodes
(points of zero probability)
Boundary surface encloses surface with a gt 90
probability of finding electron
12
electron density
wave function
radial probability distribution
13
- p orbitals Three p orbitals l 1, ml 1, 0 -
1
14
- d orbitals Five p orbitals l 2, ml 2, 1,
0 - 1, -2
15
- f orbitals Seven f orbitals l 3, ml 3, 2,
1, 0 - 1, -2. -3
16
- The three quantum numbers for an electron in a H
atom in a certain state are n 4, l 2, ml
-1. In what type of orbital is the electron
located?
17
Electron Spin
- Spectral lines observed did not have exactly the
same frequencies as those calculated by
Schrödinger. - S. Goudsmit and G. Uhlenbeck proposed electrons
have spin. Electrons behave like a spinning
sphere, like a planet rotating on its axis. - An electron has two spin states, represented by
?and ? or a and b. - Can think of these states as a counterclockwise
(?) spin or a clockwise (?), both at the same
rate. - Spin quantum number, ms, distinguishes the two
spin states - ms 1/2 ? electron
- ms - 1/2 ? electron
18
- O. Stern and W. Gerlach
19
The state of an electron in a hydrogen atom is
defined by the four quantum numbers, n, l, ml,
ms. As the values of n increases, the size of
the atom increases.
20
Many-Electron Atoms
- Electronic Structure of H atom (Z 1)
- Electron in the lowest energy level - ground
state of the atom, n 1 gt 1s orbital - Quantum numbers of this 1s electron
- n 1, l 0, ml 0, ms 1/2 or -1/2
- If the electron acquires energy, the electron can
undergo a transition to the n 2 shell and can
occupy the 2s or one of the three 2p orbitals
(for H-atom all have the same energy) - The state of an electron in a H atom is defined
by the four quantum numbers n, l, ml, ms. - As the value of n increases, the size of the atom
increases.
21
For H atom V(r ) - Z e2 / (4 p eo r) (Z 1
for H atom)
Many-electron atoms (Z gt 1) Electrons occupy
orbitals like those of a H atom. Energies of
orbitals of many electron atoms are not the same
as those for the H atom. Nuclear attraction for
electrons is greater as Z increases lowering the
electrons energy also have to account for
electron-electron repulsion.
22
- In the Schrödinger equation, V(r ) has to account
for both the nuclear-electron attraction and the
electron-electron replusion - For example for He (Z 2), V(r ) contains three
terms - V(r ) - (2 e2) / (4 p eo r1) - (2 e2) / (4 p
eo r2) e2 / (4 p eo r12) - attraction attraction
repulsion - For many-electron atoms
- The electron density of an isolated many-electron
atom is sum of the electron densities of each
electron taken individually - Every electron in an atom has a set of four
quantum numbers, n, l, ml and ms
23
- The electron-electron repulsion opposes
electron-nuclear attraction. - The repulsion shields the electron from the
full attraction of the nucleus. - Electrons feel an effective nuclear charge
which is less than the full nuclear charge. - s orbitals have a non-zero probability density at
the nucleus, penetrate through inner shells - s electrons feel stronger nuclear attraction are
tightly bound and hence lower in energy - p orbitals have zero probability density at the
nucleus less penetrating than s and hence p
electrons are higher in energy. - d orbitals less penetrating than p and hence d
electrons are higher in energy than p
24
In many electron atoms, because of shielding and
penetration effects, order of the energy of
orbitals in a given shell is typically s lt p lt d
lt f. Energies of orbitals depend on both n and l
(not just n as in the H atom)
25
Exclusion Principle
- The electronic structure of an atom determines
its chemical properties. - Electron configuration - a list of all occupied
orbitals of an atom, with the number of electrons
that each contains - Pauli Exclusion Principle No more than two
electrons may occupy any given orbital. When two
electrons occupy an orbital their spins must be
paired. - No two electrons in an atom can have the same set
of quantum numbers.
26
- (a) Spins are paired if one is ?and the other ?.
- Paired spins denoted as ?? ms of each is
different - (b) Two electrons have parallel spin if both
spins are in the same direction
27
- Building Up fill orbitals starting with the
lowest energy (aufbau principle), pairing
electrons as determined by the Pauli principle. - Order of energies of orbitals
- 5f 6d 7p 8s
- 4f 5d 6p 7s
- 3f 4d 5p 6s
- 3d 4p 5s
- 3p 4s
- 2p 3s
- 2s
- 1s