What distinguishes solids, liquids and gases from one another PowerPoint PPT Presentation
1 / 43
Title: What distinguishes solids, liquids and gases from one another
1
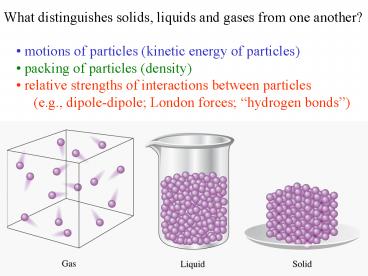
What distinguishes solids, liquids and gases from
one another?
- motions of particles (kinetic energy of
particles) - packing of particles (density)
- relative strengths of interactions between
particles - (e.g., dipole-dipole London forces
hydrogen bonds)
2
Phase Transitions or Changes of State
How is energy transferred to or from the system
during a phase transition? Which state has the
highest energy? Which state has the lowest
energy? What is the effect of a DT on the state
of matter?
3
Temperature and Kinetic Energy are positively
correlated if T ? then average KE ?
4
Why does vapor pressure increase as temperature
increases?
- particles are moving with greater energy (so
collisions are more energetic) - more particles escape liquid and become vapor
5
Dynamic equilibrium rates of vaporisation and
condensation are equal at equilibrium
6
The intermolecular forces we will consider in our
discussion of phase transitions
7
(No Transcript)
8
Dipole-dipole interactions in HCl
induced dipole or London forces between Ne
atoms
9
Ion-ion Ion-dipole Dipole-dipole
10
(No Transcript)
11
Lone pair electrons
12
(No Transcript)
13
(No Transcript)
14
See Fig 11.24 in Ebbing and Gammon
15
(No Transcript)
16
Surface tension force required to incease
surface area of some liquid
17
A (H-bonds are stronger than dipole-dipole
interactions thus, more kinetic energy is needed
to vaporize the substance)
B (weaker dipole-dipole interactions thus, less
kinetic energy is needed to vaporize the
substance)
18
Lowest (d), then (c), then (a), then (b)
(highest boiling point)
19
Why does the temperature of water remain constant
while it is boiling? If the energy supplied as
heat doesnt result in DT, what is that energy
doing?
20
Exercise 11.1 p 427 DHvap of ammonia 23.4
kJ/mol. How much heat is required to vaporize
1.00 kg of ammonia? How many grams of water
could be frozen to ice at 0 C by the evaporation
of this amount of ammonia?
How much heat is required to melt 75.0 g of ice
at -20.0 C to liquid water at 25.0 C?
21
The normal boiling point of a substance is the
temperature at which it boils when P 1 atm
A substance boils when the pressure inside a
bubble of vapor Pexternal
22
Clausius-Clapeyron Equation (derived on p 428)
ln (P2/P1) (DHvap)/R(1/T1 1/T2) Where R
8.314 J/(molK)
23
Exercise 11.2 p 429 DHvap of CS2 26.8 kJ/mol.
CS2 has a normal boiling point of 46 C. What is
Pvap of CS2 at 35 C?
At the top of Komo Kulshan (Mt. Baker) Patm 530
mmHg. At what temperature does water boil on the
top of the mountain?
24
(No Transcript)
25
Phase diagram for H2O
26
Phase diagram for CO2
27
At the critical point the densities of the
liquid and gas phases become equal, yielding
supercritical fluid. Such fluids diffuse like
gases, yet (like a liquid solvent) can solubilize
other substances (e.g., caffeine in coffee beans)
28
(No Transcript)
29
(No Transcript)
30
Two different forms of SiO2
crystalline (e.g., quartz)
amorphous (e.g., glass)
31
The crystal lattice of a crystalline solid is a
regular array of atoms or ions or molecules
How do we know this? X-ray analysis of crystals.
32
(No Transcript)
33
The unit cell is the smallest boxlike unit from
which the crystal can be reconstructed by
stacking the boxes in three dimensions.
A two-dimensional example
Nine unit cells like (D) would recreate the
lattice shown in (A) or (C)
34
Crystal unit cells
35
Cubic unit cells
36
The portions of atoms that are inside a unit
cell (example face-centered cubic)
37
(No Transcript)
38
Closest packing hexagonal (hcp) or cubic (ccp)
closest packing
39
hcp
ccp fcc
40
(No Transcript)
41
(No Transcript)
42
Based on this data, what is the value of
Avogadros number?
43
What is the atomic radius of this element?
What is the identity of this elemental substance?