Questions About Atoms PowerPoint PPT Presentation
1 / 27
Title: Questions About Atoms
1
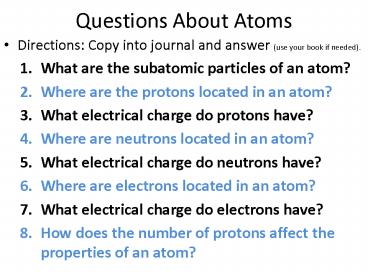
Questions About Atoms
- Directions Copy into journal and answer (use
your book if needed). - What are the subatomic particles of an atom?
- Where are the protons located in an atom?
- What electrical charge do protons have?
- Where are neutrons located in an atom?
- What electrical charge do neutrons have?
- Where are electrons located in an atom?
- What electrical charge do electrons have?
- How does the number of protons affect the
properties of an atom?
2
answers
- What are the subatomic particles of an atom?
protons, neutrons, and electrons. - Where are the protons located in an atom?
nucleus - What electrical charge do protons have?
positive - Where are the neutrons located in an atom?
nucleus - What electrical charge do neutrons have?
neutral - Where are the electrons located in an atom?
outside - What electrical charge do electrons have?
negative - How does the number of protons affect the
properties of an atom? It identifies the atom.
3
Atomic Models in HistorySemantic Feature Map
Activity
- Copy map in your Journal.
- Using the textbooks each group will research the
different types of atomic models and fill in the
table.
4
Joke
- A neutron walks into a diner and orders a glass
of orange juice at the lunch counter. When the
waiter brings the orange juice, the neutron asks,
How much do I owe you? The waiter replies, For
you, no charge.
5
Matter and Atoms Overview
6
Matter
- The term matter describes all of the physical
substances around us your table, your body, a
pencil, water, and so forth
7
Matter
- Anything that has mass and takes up space (has
volume) - Made up of different kinds of atoms
8
Matter
- Includes all things that can be seen, tasted,
smelled, or touched - Does not include heat, sound, or light
9
Matter is made of atoms
10
Models
- Models are often used for things that are too
small or too large to be observed or that are too
difficult to be understood easily
11
Models
- In the case of atoms, scientists use large models
to explain something that is very small - Models of the atom were used to explain data or
facts that were gathered experimentally. - So, these models are also theories
12
Early Models of the Atom Democritus
- Universe was made of empty space and tiny bits of
stuff - Called these tiny bits of stuff atomos
- Atoms could not be divided
13
Early Models of the Atom Lavoisier
- Demonstrated that burning wood caused no change
in mass - Law of Conservation of Matter
14
Early Models of the Atom Dalton
- All elements are composed of indivisible
particles. - Atoms of the same element are the same
- Atoms of different elements are different.
- Compounds consisted of atoms of different
elements combined together
15
Early Models of the AtomThomson
- Plum pudding model
- Atom made of a positively charged material with
the negatively charged electrons scattered
through it.
16
Early Models of the Atom Rutherford
- Mostly empty space
- Small, positive nucleus
- Contained protons
- Negative electrons scattered around the outside
17
Early Models of the Atom Bohr
- Electrons move in definite orbits around the
nucleus
18
Early Models of the Atom Chadwick
- Discovered the neutron
19
Modern Model of the AtomThe electron cloud
- Sometimes called the wave model
- Spherical cloud of varying density
- Varying density shows where an electron is more
or less likely to be
20
Atomic Structure
- Nucleus
- Protons
- Neutrons
- Electrons
21
Atomic Structure
- Electrons
- Tiny, very light particles
- Have a negative electrical charge (-)
- Move around the outside of the nucleus
22
Atomic Structure
- Protons
- Much larger and heavier than electrons
- Protons have a positive charge ()
- Located in the nucleus of the atom
23
Atomic Structure
- Neutrons
- Large and heavy like protons
- Neutrons have no electrical charge
- Located in the nucleus of the atom
24
Atomic Structure
25
Describing Atoms
- Atomic Number number of protons
- In a neutral atom, the of protons the of
electrons
26
Describing Atoms
- Atomic Mass Number - equal to the number of
protons plus neutrons.
27
Describing Atoms
- Atomic Weight - average mass of the naturally
occurring isotopes of an element .