Building A Toolset PowerPoint PPT Presentation
1 / 34
Title: Building A Toolset
1
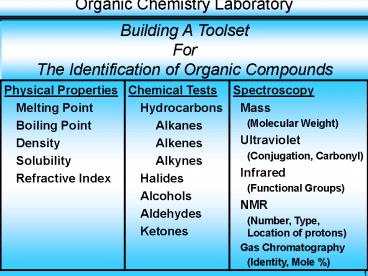
Building A Toolset For The Identification of
Organic Compounds
2
- Study of the Interaction of Electromagnetic
Radiation (Energy) and Matter - When Energy is applied to matter it can be
- Absorbed
- Emitted
- Cause a chemical change (reaction)
- Transmitted.
- Electromagnetic Spectrum
Cosmic ? (Gamma) X-Ray Ultraviolet Visible Infr
ared Microwave Radio
3
- Spectroscopy Types
- Mass Spectrometry (MS) Hi-Energy Electron
Bombardment - Use Molecular Weight, Presence of Nitrogen,
Halogens - Ultraviolet Spectroscopy (UV) Electronic Energy
States - Use Presence of Conjugated Molecules Carbonyl
Group - Infrared Spectroscopy (IR) Vibrational Energy
States - Use Functional Groups Compound Structure
- Nuclear Magnetic Resonance Spectroscopy (NMR)
Nuclear Spin States - Use The number, type, and relative position of
protons (Hydrogen nuclei) and Carbon-13
nuclei
4
- High energy electrons bombard organic molecules
breaking some or all of the original molecules
into fragments. - The process usually removes a single electron to
produce a positive ion (cation radical) that can
be separated in a magnetic field on the basis of
the mass / charge ratio. - Removal of the single electron produces a charge
of 1 for the cation. - Thus, the cation represents the Molecular Weight
of the original compound or any of the fragments
that are produced. - The mass spectrum produced is a plot of relative
abundance of the various fragments versus the
Mass / Charge (M/Z) ratio. - The most intense peak is called the Base Peak,
which is arbitrarily set to 100 abundance all
other peaks are reported as percentages of
abundance of Base Peak.
5
Typical Mass Spectrum
6
- Molecular Ion Peak (M) Molecular Wgt of
Original Molecule - Largest mass/charge ratio and it is always the
last peak on the right side of spectrum - Molecular Ion Peak may or may not be the base
peak! - The Molecular Ion Peak(s) abundance can be quite
small. - The presence of Nitrogen in the compound If the
Mass / Charge (m/z) ratio for the Molecular Ion
peak is Odd, then the molecule contains an Odd
number of Nitrogen atoms, i.e., 1, 3, 5, etc. - Note An Even value for the Mass / Charge
ratio could represent a compound with an even
number of Nitrogen atoms, i.e., 0, 2, 4 etc., but
the actual presence of Nitrogen in the compound
is not explicitly indicated as it is with an
Odd value for the ratio.
7
- Most elements exist in several isotopic forms
- Ex. 1H1, 2H1, 12C6, 13C6, 35Cl17, 37Cl17, 79Br35,
81Br35 - Each peak in a Mass Spectrum represents the
Integral Molecular Weight of the fragment not
the Average Molecular Weight usually reported - Integral Molecular Weight represents an
integral number of protons and neutrons, i.e.,
a specific isotope. - Average Molecular Weight represents the average
molecular weight of All isotopes present. - Thus, all peaks in a Mass Spectrum for a given
fragment reflect the naturally occurring isotopic
mixture of the elements in the fragment.
8
- The Presence of Chlorine in a Compound
- The two (2) principal Chlorine Isotopes in nature
are Cl-35 and Cl-37 (2 additional Neutrons in
Cl-37) - The relative abundance ratio of Cl-35 to Cl-37 is
100 32.6 or 75.8 24.2 or ?
3 1 - Therefore, a Molecule containing a single
Chlorine atom will show two Mass Spectrum
Molecular Ion peaks, one for Cl-35 (M) and one
for Cl-37 (M2) - Note M2 denotes 2 more neutrons than M
- Based on the natural abundance ratio of 100 /
32.6 (about 31), the relative intensity (peak
height) of the Cl-35 peak will be 3 times the
intensity of the Cl-37 peak.
9
- The presence of Bromine in a compound
- The two (2) principal Bromine Isotopes in nature
are Br-79 and Br-81 (2 additional Neutrons in
Br-81) - The relative abundance ratio of Br-79 to Br-81 is
100 97.1 or 50.5 49.5 or ? 1
1 - Molecules containing a single Bromine atom will
also show two molecular ion peaks one for Br-79
(M) and one for Br-81 M2). - Based on the natural abundance ratio of 100 /
97.1 (about 11), the relative intensity of the
Br-79 peak will be about the same as the Br-81
peak. - Note Fluorine exists in nature principally as a
single isotope - 19F9 - Therefore, single Molecular Ion peak
(assuming no other Halogens present.
10
- Compounds containing two (2) Chlorine atoms will
produce three (3) Molecular Ion peaks
representing the 3 possible isotope combinations
available - 35Cl17 35Cl17 (Rel Peak Intensity - 100.0)
- 35Cl17 37Cl17 (Rel Peak Intensity -
65.3) - 37Cl17 37Cl17 (Rel Peak Intensity -
10.6) - Compounds containing three (3) Chlorine atoms
will produce four (4) Molecular Ion peaks
representing the 4 possible isotope combinations
available - 35Cl17 35Cl17 35Cl17 (Rel Peak Intensity
- 100.0) - 35Cl17 35Cl17 37Cl17 (Rel Peak Intensity
- 97.8) - 35Cl17 37Cl17 37Cl17 (Rel Peak Intensity
- 31.9) - 37Cl17 37Cl17 37Cl17 (Rel Peak Intensity
- 3.5)
11
- UV-Visible Spectrum 200 nm 700 nm
- Most organic molecules and functional groups are
transparent in the Ultraviolet and Visible
portions of the electromagnetic spectrum. - Thus
- Absorption Spectroscopy in theUltraviolet /
Visible Range is of Limited Utility - In the case of ultraviolet and visible
spectroscopy, the energy absorption transitions
that occur are between electronic energy levels
of valence electrons, that is, orbitals of lower
energy are excited to orbitals of higher energy. - Thus, UV / Visible spectra are often called
Electronic Spectra
12
- Molecules have many excited modes of vibration
and rotation at room temperature. The rotational
and vibrational levels are superimposed on the
electronic levels - Electron transitions may occur from any of
several vibrational and rotational states of one
electronic level to any of several vibrational
and rotational states of a higher electronic
level. - Thus, the UV spectrum of a molecule consists of a
broad band of absorption centered near the
wavelength of the major transition
13
- UV Spectroscopy is generally limited to the
determination of the presence of a Conjugated
Unsaturated System and Carbonyl Groups. - Conjugated Unsaturated Systems
- Conjugated unsaturated systems are molecules with
two or more double or triple bonds each
alternating with a single bond.
Ex. CH2CH ? CHCH2 - Conjugated unsaturated systems are species that
have delocalized ? bonds, i.e. a p-orbital on an
atom adjacent to a double bond producing ? ? ?
transitions. - Single electron as in the allyl radical
(CH2CH?CH2) - Vacant p orbital as in allyl cation (CH2CH?CH2)
- P orbital of another double bond (CH2CH ?CHCH2
14
- Compounds whose molecules contain conjugated
multiple bonds have absorption maxima at
wavelengths longer than 200 nm, i.e. in the UV
range. - Conjugated systems absorb strongly in the UV /
Visible portion of the electromagnetic spectrum,
therefore they can be investigated with
Ultraviolet Spectroscopy. - More complicated alkenes (carbon-carbon double
bond) and nonconjugated dienes usually have
absorption maxima below 200 nm, i.e. do not
absorb in the UV range).
15
- Carbonyl Compounds
- Compounds with carbon-oxygen double bonds
(carbonyl) also absorb light in the UV region. - The carbonyl excitation process involves movement
of an electron from one of the unshared
(nonbonding) pair to the ? orbital of the
carbon-oxygen double bond.
Transitions - n ? ? - Non-bonding electrons, such as those in a
carbonyl group (and some alkyl halides), will
absorb in the UV region, but at lower intensity
than conjugated systems. - Carbonyl absorption in the UV does not require
additional conjugation in the molecule. - If a molecule does not absorb in the UV, then it
does not contain a conjugated system of
alternating double bonds or a carbonyl group
16
Ultraviolet / Visual Spectrophotometers
- Produce an absorption spectrum, which is a plot
of the wavelength in nanometers (nm) over the
entire Ultraviolet / Visible region versus the
absorbance (A) of the radiation at each
wavelength. - Note Absorption by the solvent is measured
first and then electronically
subtracted from the solvent / sample
mixture. - A log (Ir / Is)
- Is Intensity of Sample
Beam - Ir Intensity of Reference
Beam - The Wavelength of Maximum Absorption ( ?max ) is
obtained from the Absorption Spectrum
17
- Molar Absorptivity (?) - also called the Molar
Extinction Coefficient - is a measure of the
strength or intensity of the absorption. - It is the proportionality constant relating the
observed absorbance (A) at a particular
wavelength to the molar concentration (C) of the
sample and the length (l) of the path of the
light beam through the sample cell (cm). - A ? C
l - ? A / (C
l )
2,5-Dimethyl-2,4-Hexadiene (in
Methanol) Wavelength of Maximum Absorbance
(?max) 242.5 nm Molar Absorptivity ( ? )
13,100 M-1 cm-1 (Log ? 4.1)
18
Conjugated systems show large values of ? ?
1000 100,000 (Log ? 3 - 5) Carbonyl
compounds show smaller values of ? ? 10 100
(Log ? 1 - 2)
19
Wavelength of Maximum Absorbance ?max 230
nm Molar
Absorptivity ? 15,000 cm-1
Log ? 4.2 ?
Conjugated Molecule (Benzene Ring)
20
Infrared Radiation That part of the
electromagnetic spectrum between the visible and
microwave regions 0.8 ?m (12,500 cm-1) to 50
?m (200 cm-1). Area of Interest in Infrared
Spectroscopy The Vibrational portion of infrared
spectrum 2.5 ?m (4,000 cm-1) to 25 ?m (400
cm-1) Radiation in the vibrational infrared
region is expressed in units called wavenumbers (
) Wavenumbers are expressed in units of
reciprocal centimeters (cm-1) i.e. the reciprocal
of the wavelength (?) expressed in centimeters.
(cm-1) 1 / ?
(cm)
21
- Molecular Vibrations
- Absorption of infrared radiation corresponds to
energy changes on the order of 8-40 KJ/mole (2-10
Kcal/mole - The frequencies in this energy range correspond
to the stretching and bending frequencies of the
covalent bonds with dipole moments. - Stretching (requires more energy than bending)
- Symmetrical
- Asymmetrical
- Bending
- Scissoring (in-plane bending)
- Rocking (in-plane bending)
- Wagging (out-of-plane bending)
- Twisting (out of plane bending)
22
- No two molecules of different structure will have
exactly the same natural frequency of vibration,
each will have a unique infrared absorption
pattern or spectrum. - Two Uses
- IR can be used to distinguish one compound from
another. - Absorption of IR energy by organic compounds will
occur in a manner characteristic of the types of
bonds and atoms in the functional groups present
in the compound thus, infrared spectrum gives
structural information about a molecule. - The absorptions of each type of bond (NH, CH,
OH, CX, CO, CO, CC, CC, CC, CN, etc.) are
regularly found only in certain small portions of
the vibrational infrared region, greatly
enhancing analysis possibilities.
23
The Infrared Spectrum A plot of absorption
intensity ( Transmittance) on the y-axis vs.
frequency (wavenumbers) on the x-axis.
Methyl IsopropylKetone
Aliphatic C-H Stretch
CH3
CO Carbonyl
24
Principal Frequency Bands (from left to right in
spectrum) OH 3600 cm-1 (Acids - Very Broad,
Alcohols - Broad) NH 3300-3500 cm-1 (2, 1, 0
peaks 1o, 2o, 3o) CN 2250 cm-1 (Nitrile) CC 21
50 cm-1 (Acetylene) CO 1685-1725
cm-1 (Carbonyl) CC 1650 cm-1 (Alkene) CC 1450-16
00 (Aromatic - 4 absorptions) CH2 1450
cm-1 (Methylene) CH3 1375 cm-1 (Methyl) CO 900-11
00 cm-1 (Alcohol, Acid, Ester, Ether,
Anhydride) CH Sat Alkanes Right side of 3000
cm-1 CH Unsat Alkenes Left side of 3000 cm-1,
1650 cm-1 CH Aromatic Verify at 16672000
cm-1, 1450-1600-1
25
Analyzing the Spectrum A Suggested Approach
- Step 1. Check for the presence of the Carbonyl
group (CO) at 1715 cm-1. If molecule is
conjugated, the strong (CO) absorption will be
shifted to the right by 30 cm-1, i.e.
1685 cm-1 - If the Carbonyl absorption is present, check for
- Carboxylic Acids - Check for OH group (broad
absorption near 3300-2500 cm-1) - Amides - Check for NH group (1 or 2
absorptions near 3500 cm-1) - Esters - Check for 2 C-O group (medium
absorptions near 1300-1000 cm-1) - Anhydrides - Check for 2 CO absorptions near
1810 and 1760 cm-1 - Aldehydes - Check for Aldehyde CH group (2 weak
absorptions near 2850 and 2750 cm-1) - Ketones - Ketones (The above groups have been
eliminated)
26
- Step 2. - If the Carbonyl Group is Absent Check
for Alcohols, Amines, or
Ethers. - Alcohols Phenols - Check for OH group (Broad
absorption near 3600 - 3300 cm-1 Confirm
present of CO near 1300 - 1000 cm-1 - Amines - Check for NH stretch (Medium
absorptions) near 3500 cm-1 - Primary Amine - 2 Peaks
- Secondary Amine - 1 Peak
- Tertiary Amine - No peaks
- N-H Scissoring at 1560 - 1640 cm-1
- N-H Bend at 800 cm-1
- Ethers - Check for CO absorption near 1300 -
1000 cm-1 and absence of OH - Esters Unbalanced Ethers will show 2 CO
groups
27
- Step 3. Refine the Structure Possibilities by
Looking for Double Bonds,
Triple Bonds and Nitro Groups - Double Bonds - Unsaturated CC (and CC) stretch
show absorptions on the left side of 3000 cm-1 - Alkene CC weak absorption near 1650 cm-1
- Aromatic CC (4 absorptions 1450-1600 cm-1)
- (Verify Aromatic at 1667 2000 cm-1)
- Triple Bonds C N Nitrile - medium, sharp
absorption (stretch near 2250 cm-1) R
C C R Alkyne - weak, sharp absorption
(stretch) near 2150 cm-1 R C C H
Terminal Acetylene
(stretch at 3300 cm-1) - Nitro Groups - Two strong absorptions 1600 1500
cm-1 and 1390 - 1300 cm-1
28
- Step 3 (Cont)
- Aromatic Ring Absorptions
- If the absorptions on the left side of 3000 cm-1
are due to the presence of aromatic (benzene
ring) CC bonds, the aromaticity and subsequent
ring substitution patterns can be verified and
further elucidated in three other regions - The presence of 1-4 weak absorptions in the
Overtone region (1667 2000 cm-1) - The presence of 1-3 strong absorptions in the
out-of-plane (OOP) region (900 - 690 cm-1) - Four medium to strong absorptions in region 1650
- 1450 cm-1 - The relative shapes and numbers of the Overtone
and OOP absorptions can be used to tell whether
the aromatic ring is monosubstituted or di-,
tri-, tetra-, penta-, or hexa-substituted. - In addition, the ortho-, meta-, para-
substitutions can also be distinguished for the
di-substituted isomers.
29
- Step 3 (Cont)
- Aromatic Ring Absorptions (Cont)
- The unsaturated C-H Out-of-Plane (OOP) bending
absorptions in the region 900 690 cm-1 can also
be used to determine the type of ring
substitution. - The number of absorptions and their relative
positions are unique to each type of
substitution. - Although these absorptions are in the
Fingerprint region they are particularly
reliable for rings with Alkyl group
substitutions. - They are less reliable for Polar substituents.
30
(No Transcript)
31
Step 4. If none of the above apply then the
compound is most likely a Hydrocarbon.Generally,
a very simple spectrum Hydrocarbons - Check for
saturated Alkane absorptions near right side of
3000 cm-1
32
IR Analysis Scheme
Carbonyl (CO) _at_ 1715-1685 (Conjugation moves
absorption to right 30 cm-1
Yes
No
Acid Ester Amide Anhydride Aldehyde Ketone
Alcohol Amine Ether
Saturation lt 3000 cm-1
Unsaturation gt 3000 cm-1
Alkanes -C-H Methylene -CH2 Methyl -CH3
Alkenes (Vinyl) -CC Aromatic -CC
Nitriles
Nitro
Hydrocarbons
33
Carbonyl (CO) is Present Acid - Broad OH
Absorption _at_ 3300-2500 cm-1 Ester - C-O
Absorption _at_ 1300-1000 cm-1 Amide - NH Absorption
_at_ 3500 cm-1 (1 or 2 peaks) Anhydride - 2 CO
Absorptions 1810 1760 cm-1 Aldehyde - Aldehyde
C-H Absorptions _at_ 2850 2750 cm-1 Ketone - None
of the above except CO
Carbonyl is Absent Alcohol - Broad OH absorption
_at_ 3300 - 3000 cm-1 Also C-O
absorption _at_ 1300 - 1000 cm-1 Amine - 1 to 2
equal NH absorptions _at_ 3500 cm-1 Ether - C-O
absorption _at_ 1300 - 1000 cm-1
34
Saturation
Alkanes -C-H Stretch several absorptions to
right of 3000 cm-1 Methylene -CH2 1450
cm-1 Methyl -CH3 1375 cm-1
Unsaturation
Double Bonds C-H Stretch several absorptions
to left of 3000 cm-1 OOP bending at 1000
650 cm-1 Alkenes (Vinyl) -CC- Stretch (weak) _at_
1675 1600 cm-1
Conjugation moves absorption to
the right Alkynes CC-H Terminal Acetylene
Stretch at 3300 cm-1 Alkynes (Acetylenes) -CC Str
etch _at_ 2150 cm-1
Conjugation moves absorption to the
right Aromatic C-H Stretch absorptions also to
left of 3000 cm-1 OOP bending at 900 690
cm-1 OOP absorption patterns allow
determination of ring substitution (p.
902 Pavia text) -CC 4 Sharp absorptions (2
pairs) _at_ 1600 1450 cm-1 Overtone absorptions
_at_ 2000 1667 cm-1 Relative shapes and numbers
of peaks permit determination of ring
substitution pattern (p. 902 Pavia text).