Chapter 5 Stereochemistry - PowerPoint PPT Presentation
1 / 36
Title:
Chapter 5 Stereochemistry
Description:
chiral - the mirror image of an object produces another object ... enatiomer of gauche. butane?? Chapter 5. 19. An achiral intermediate or transition state that is ... – PowerPoint PPT presentation
Number of Views:146
Avg rating:3.0/5.0
Title: Chapter 5 Stereochemistry
1
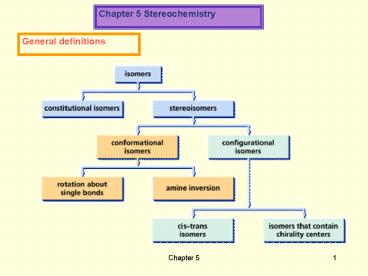
Chapter 5 Stereochemistry
General definitions
2
(No Transcript)
3
chiral - the mirror image of an object produces
another object which is not superimposible on the
original. From the Greek word for
handed. achiral - something that is not chiral
enantiomers - pairs of molecules that are mirror
images of each other and are nonsuperimposible
4
- chiral molecules
- 1. two representatives -
- 3-methylhexane
- 3-methylpentane
5
3-methylpentane is achiral
6
3-methylhexane is chiral
7
Generalization
Compounds with four different groups around a
carbon atom generate a chiral molecule. The
carbon atom with the four different groups is
said to be a chirality center or a stereogenic
center.
8
2. Elements necessary for an achiral
molecule. a. an internal mirror plane of symmetry
internal mirror plane
9
b. inversion center
10
3. Telling the difference between two
enantiomers a. plane polarized light
11
If one enantiomer rotates plane polarized light
by x in a clockwise direction, then the other
enatiomer will rotate plane polarized light by x
in the counterclockwise direction. d -
dextrorotatory - () enantiomer ? rotate
clockwise l - levorotatory - (-) enantiomer ?
rotate counterclockwise
racemic mixture - a 5050 mixture of two
enantiomers for example the (d,l) or
(,-)-3-methylhexane mixture
12
b. another chiral molecule that interacts with
the two enantiomers
13
the most important example is enzymes in cells
R ? good - morning sicknes S ? bad - birth
defects
14
4. Absolute configuration - the
Cahn-Ingold-Prelog scheme a. assign a priority
number to the four groups around the chiral
center 1highest, 4lowest priority i. atomic
number of the atom directly connected to the
chiral center ii. in case of ties- go to
next set of atoms
15
one atom of higher priority takes precedence over
any number of others
note
iii. for multiple bonds - break and add imaginary
atoms as follows
16
b. Orient the molecule so the group with lowest
priority, 4 Is in back of the plane of the paper.
Now rotate from highest,1 to 2 to 3.
If the rotation is clockwise then the
configuration is R - rectus If the
rotation is counterclockwise, then the
configuration is S - sinister
17
(No Transcript)
18
5. conformationally mobile systems
Can we make one enatiomer of gauche butane??
19
An achiral intermediate or transition state
that is energetically accessible always leads to
racemization.
20
Another example The sad story of
cis-1,2-dimethylcyclohexane
e, a
a, e
enantiomers
21
B. Fischer Projections - a lazy mans way to
handle stereochemistry - a poor mans
representation of
3 - D
BE VERY CAREFUL WITH THIS!!!!!
- General construction
- convention 1
22
Mirror plane
23
NOTE when viewing from the backside, the sense
of rotation reverses!
24
Lets flip the structure
- So the moral of this story is as follows
- A 90 rotation or flipping the structure around
either axis will cause it to be converted into
its enantiomer. - 2. Rotation by 180 keeps the same configuration.
25
The virtue of Fischer projections is that they
can be easily used for molecules with more than
one chiral center. Lets take 2, 3-dibromobutane
as an example
Is it chiral?
This molecule is still not superimposible on the
first one by sliding it, so it must be an
enantiomer
mirror plane
26
What about this configuration for
2,3-dibromobutane??
mirror plane
This molecule is superimposible on the first one
by sliding it, so it must be identical to the
first.
Therefore this molecule is NOT chiral!
27
2. Assigning R, S configurations - within the
Fischer projection
Convebtion 2 a. Always put the lowest priority
group in the horizontal position. b. Going from
priority 1 to 2 to 3, a rotation counterclockwise
is R and a rotation clockwise is S.
THIS IS EXACTLY OPPOSITE TO DOING THE
CONFIGURATION ASSIGNMENT IN THE ACTUAL 3-D
SPACE!!!!
Lets take a few cases---
28
therefore
and
Now - what is the relationship between the R,R
and S,S configurations and the R,S one??
29
(No Transcript)
30
C. Compounds with more than one center of
chirality. For 2,3-dibromobutane there are three
different steroisomers, in general terms we
have
So there are 2n possible stereoisomers if
there are n chiral centers. However, this is not
true for molecules that have an internal mirror
plane of symmetry -
31
Internal mirror plane of symmetry
In reality a center of inversion
Fischer projections are most useful for sugars
glucose
32
D. Resolution of enantiomers - forming two (or
more) chiral centers is the whole idea how we can
tell or separate one enatiomer from another
1. They form diasteromeric complexes with the
chiral agent i ) Consider AR and AS to be two
enantiomers. ii ) BR may be a chiral solvent, a
chiral substrate, or even just plane polarized
light that propagates in a clockwise (or an
anticlockwise) direction. AR and AS BR ?
ARBR and ASBR these are diastereomers
2. Diastereoisomeric interactions are used to
separate enantiomers. If, for example, A is a
racemic acid and B is a chiral base (a natural
product), the salts that are produced can be
expected to have different solubility properties.
33
(No Transcript)
34
Pharmaceutical Industry Marketed
Compounds Total 1850
Natural Semisynthetic 520
Synthetic 1370
achiral 6
chiral 517
achiral 788
chiral 528
racemate 8
single enantiomer 509
racemate 467
single enantiomer 61
35
turpentine oder from fir trees
orange oder from oranges
caraway oil
oil of spearmint
36
E. Summary
- Definitions
- a. chiral/ achiral
- b. diastereomer/ enantiomer
- c. configuration/ conformation
- d. mirror plane/ center of inversion
- 2. Absolute configuration know
Cahn-Ingold-Prelog rules - a. determine priority of groups around chiral
center - b. determine R, S configuration
- 3. More than one center of chirality
- a. Fischer projections
- i) manipulations, ie inverting, flipping,
rotating - ii) determining R, S
- b. Diastereomers - resolution of enantiomers