What is a Carbon Nanotube? - PowerPoint PPT Presentation
1 / 17
Title:
What is a Carbon Nanotube?
Description:
What is a Carbon Nanotube? Start with Carbon Graphite C60 Single Wall Carbon Nanotubes Multi Wall Carbon Nanotubes * For semiconducting * ? Why no metallic? – PowerPoint PPT presentation
Number of Views:1119
Avg rating:3.0/5.0
Title: What is a Carbon Nanotube?
1
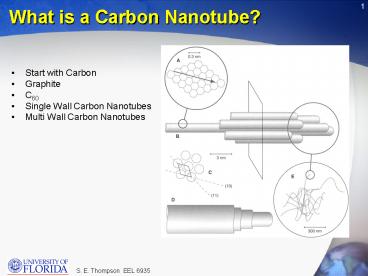
What is a Carbon Nanotube?
- Start with Carbon
- Graphite
- C60
- Single Wall Carbon Nanotubes
- Multi Wall Carbon Nanotubes
2
Start with Carbon
- Carbon contains six electrons
- (1s)2, 2s, 2px,2py, 2pz
- 1s quantum number N1 (2 electrons)
- N2 , four electrons
- s orbital spherically symmetric about nucleous
- p directed charge distribution
- s and p form chemical bond
- Y s lp
- Solid carbon two main structures
- Diamond sp3 109 degree bonds
- Graphitic sheet sp2 120 degree bonds. Each bond
in same plane - Graphite s, px, py
- Sheets held together by weaker van der Waals
Forces
3
Sp3 and sp2 Bonds
4
Discovery of C60
- Soccer ball-like molecule containing 60 carbon
atoms - Motivated by understanding light transmission
through interstellar dust - Optical extinction absorption and scattering of
light from interstellar dust - C60 envisioned by theoretical chemist
- High powered pulsed laser simulate conditions of
hot carbon - Prof. Richard Smalley (Rice) observed mass number
720 mass spectrometer (carbon mass 12) - Smalley won Nobel prize
5
C60
Ref Intro to Nanotechnology
6
Closed Network From Other Atoms
Ref Intro to Nanotechnology
7
Extension of C60, C70, C80
End closed
8
Formation of an Armchair Nanotube
Chiral vector is bent
2D Graphene Sheet
armchair (n,n)
9
Unit Cell of 2D Graphene
(a) The unit cell and (b) Brillouin zone of
two-dimensional graphite are shown as the dotted
rhombus and shaded hexagon, respectively. ai, and
bi, (i 1, 2) are unit vectors and reciprocal
lattice vectors, respectively. Energy dispersion
relations are obtained along the perimeter of the
dotted triangle connecting the high symmetry
points, ?, K and M.
10
Roll Carbon Nanotube from Graphite
The unrolled honeycomb lattice of a nanotube,
showing the unit vectors a1 and a2 for the
graphene sheet. When we connect sites O and A,
and B and B, a nanotube can be constructed. OA
and OB define the chiral vector Ch and the
translational vector T of the nanotube,
respectively. The rectangle OABB defines the
unit cell for the nanotube. The figure
corresponds to Ch (4, 2)
11
Constructing Nanotubes from a Graphene Sheet
Roll-up vector
a1
a2
12
3D Examples of 3 Types of Nanotubes
13
Some More Properties of Nanotubes
- 1-50nm in diameter
- 10 - 100 micrometer long
- End capped with half fullerene molecule
- Single and multi-wall nanotubes
- Chirality refers to how the tubes are rolled
- One-third metallic, two-thirds semiconducting
- Energy gap 1/(diameter of tube)
- Diameter of tube increases, bandgap decreases
14
Metallic and Semiconducting CNT
15
Examples of Band Structures
One-dimensional energy dispersion relations for
(a) armchair (5, 5), (b) zigzag (9, 0), and (c)
zigzag (10, 0) carbon nanotubes.
16
Bandgap of Semiconductor Tube
17
Observed Nanotube Species