CELLULE - PowerPoint PPT Presentation
1 / 14
Title:
CELLULE
Description:
Macrophage/CD4+ CCR5+ Th1 lymphocytes cross-talk during MTB/HIV ... Reinforced HIV replication and mycobacterial dissemination IL-1b/TNF-a HIV MTB TLR-2 La ... – PowerPoint PPT presentation
Number of Views:59
Avg rating:3.0/5.0
Title: CELLULE
1
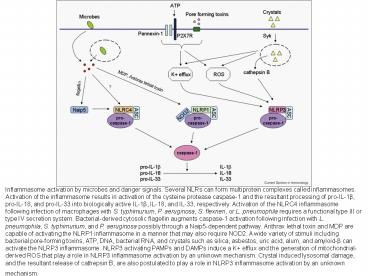
Inflammasome activation by microbes and danger
signals. Several NLRs can form multiprotein
complexes called inflammasomes. Activation of the
inflammasome results in activation of the
cysteine protease caspase-1 and the resultant
processing of pro-IL-1ß, pro-IL-18, and pro-IL-33
into biologically active IL-1ß, IL-18, and IL-33,
respectively. Activation of the NLRC4
inflammasome following infection of macrophages
with S. typhimurium, P. aeruginosa, S. flexneri,
or L. pneumophila requires a functional type III
or type IV secretion system. Bacterial-derived
cytosolic flagellin augments caspase-1 activation
following infection with L. pneumophila, S.
typhimurium, and P. aeruginosa possibly through a
Naip5-dependent pathway. Anthrax lethal toxin and
MDP are capable of activating the NLRP1
inflammasome in a manner that may also require
NOD2. A wide variety of stimuli including
bacterial pore-forming toxins, ATP, DNA,
bacterial RNA, and crystals such as silica,
asbestos, uric acid, alum, and amyloid-ß can
activate the NLRP3 inflammasome. NLRP3 activating
PAMPs and DAMPs induce a K efflux and the
generation of mitochondrial-derived ROS that play
a role in NLRP3 inflammasome activation by an
unknown mechanism. Crystal induced lysosomal
damage, and the resultant release of cathepsin B,
are also postulated to play a role in NLRP3
inflammasome activation by an unknown mechanism.
2
(No Transcript)
3
Figure 1. Regulation of the inflammasomes by host
factors and pathogen effectors. Inflammasomes are
activated in a two-step process beginning with
PRR-mediated induction of inflammasome components
and pro-IL-1ß production through NF-?B, followed
by a second signal that activates the
inflammasome and caspase-1 catalysis. This
process can be regulated at multiple steps by
host proteins that function as positive
regulators (green) or inhibitors (orange) or
targeted by pathogen effectors (red). Host COPs
and POPs, the poxvirus proteins M13L and gp013L,
and the anti-apoptotic factors Bcl-2 and Bcl-XL
inhibit inflammasome assembly. Caspase-1
activation is inhibited by caspase-12, and
multiple pathogen effectors, while murine
caspase-11 and human caspase-5 are required for
caspase-1 activation in response to certain
stimuli. Type I IFN is required for AIM2
inflammasome activation in response to cytosolic
DNA. The IL-1ß and IL-18 pathways are also highly
regulated. Endogenous IL-1 receptor antagonist
(RA) prevents IL-1 signaling by binding to the
IL-1 receptor, while the vaccinia virus proteins
B15R and Molluscum contagiosum poxvirus MC53L and
MC54L can bind and inhibit IL-1 and IL-18,
respectively.
4
(No Transcript)
5
Figure 2. Microbial activation of the
inflammasomes. Pathogenic microorganisms activate
the inflammasomes through multiple agonists and
pathways. S. typhimurium, L. pneumophila, and M.
tuberculosis reside within the host cell
phagosome and are capable of activating
inflammasomes through secreted flagellin,
effectors, or undefined NLRP3 agonists. F.
tularensis and L. monocytogenes, which escape the
phagosome activate AIM2 that senses cytosolic
DNA. B. anthracis lethal toxin activates the
NLRP1 inflammasome. C. albicans and hemozoin
activate NLRP3 through SYK signaling.
Viral-mediated inflammasome activation is heavily
dependent on the detection of nucleic acids by
NLRP3, AIM2, and RIG-I. Dotted lines indicate
signaling through an unknown mechanism.
6
Macrophage/CD4 CCR5 Th1 lymphocytes cross-talk
during MTB/HIV coinfection (1)
7
Macrophage/CD4 T lymphocytes cross-talk during
MTB/HIV coinfection (2)
Reinforced HIV replication and mycobacterial
dissemination
8
(No Transcript)
9
La fagocitosi di corpi apoptotici ha sempre un
esito anti-infiammatorio? Dipende
10
The four known inflammasomes. (a) The Nlrp3
inflammasome activates caspase-1 by recruiting
ASC. (b) The Nlrp1 inflammasome has a FIIND and
CARD domain in addition to its LRR, NACHT and
Pyrin domains and can recruit caspase-5. (c) The
Nlrc4 inflammasome has a CARD domain that can
directly recruit procaspase-1. (d) AIM2 consists
of a Pyrin and DNA-binding HIN domain that forms
a complex with ASC and caspase-1. ASC,
apoptosis-associated speck-like protein
containing a CARD FIIND, function to find
domain LRR, leucine-rich repeat AIM2, absent
from melanoma 2 MDP, muramyl dipeptide.
11
Differential IL-1ß secretion pathways in
monocytes and macrophages. Caspase-1 is
constitutively active in monocytes, and these
cells release mature IL-1ß after a single
stimulation with a TLR ligand. In contrast,
macrophages need two signals for IL-1ß secretion
one, such as a TLR-ligand, that induces IL-1ß
transcription, and a second signal that induces
inflammasome activation.
12
IL-1ß processing in acute and chronic stages of
inflammation. Neutrophils are the major source
for processing IL-1ß via PR3 during acute
inflammatory conditions. In chronic stages of
inflammation when monocytes and macrophages play
a more dominant role, caspase-1 and inflammasome
activation become more important for the
production of mature IL-1ß.
13
Death and inflammation how caspase-1 (ICE)
activates IL-1ß and IL-18 to induce innate and
adaptive immunity and how this inflammation may
be modulated. Apoptotic death induced by a
variety of factors (such as ligation of Fas by
its ligand) triggers a caspase cascade that can
include ICE. Caspase-11 may be involved in the
activation of ICE or may form a complex with it
16. Activated ICE is then capable of cleaving
the pro-forms of IL-1ß and IL-18, which in turn
trigger other proinflammatory cytokines. There is
also evidence that activated ICE plays a role in
the induction of apoptosis under some conditions
(hence the question mark) 19. During poxviral
infection, CrmA can block the activity of ICE. A
number of other factors may regulate or even
abrogate the activation of innate and adaptive
immune responses ICE, or its substrates IL-1ß
and IL-18, may not be expressed by the dying
cell IL-1-receptor antagonist (IL-1RA) may
modulate or block the effects of IL-1ß and
macrophages that take up apoptotic cells through
the phosphatidylserine (PS) receptor do not
produce TNF-a in response to lipopolysaccharide
(LPS) but may produce the anti-inflammatory
cytokine, TGF-ß
14
Figure 1 -?-glutyamyl-meso-DAP (iE-DAP) and
muramyl dipeptide (MDP), respectively, leading to
recruitment of the adaptor proteins RICK and
caspase recruitment domain 9 (CARD9).
Subsequently, both TLRs and NOD1/NOD2 signaling
pathways recruit TAK1, which mediates the
activation of nuclear factorkappa B (NF-?B) and
mitogen-activated protein kinases (MAPKs),
resulting in the transcriptional upregulation of
proinflammatory genes. (c) Activation of NLRs by
microbial or endogenous molecules in the cytosol
results in the formation of caspase-1-activating
inflammasomes. Activation of caspase-1 induces
processing of the interleukin-1-beta (IL-1ß)
precursor and secretion of the mature cytokine.
Abbreviations ERK, extracellular
signalregulated protein kinase IKK, I-kappa-B
kinase JNK, c-Jun N-terminal kinase MKK, MAP
kinase kinase NEMO, NF-?B essential modulator.