Structural Theory - PowerPoint PPT Presentation
Title:
Structural Theory
Description:
Lewis Theory Valence Bond ... electron pair Figure 1.2 A periodic table of the common elements seen in organic chemistry So let s write some Lewis Structures CCl4 ... – PowerPoint PPT presentation
Number of Views:102
Avg rating:3.0/5.0
Title: Structural Theory
1
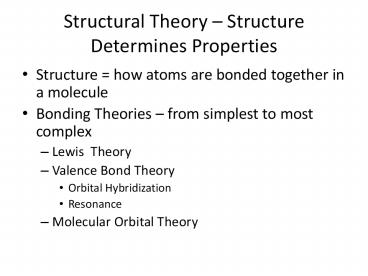
Structural Theory Structure Determines
Properties
- Structure how atoms are bonded together in a
molecule - Bonding Theories from simplest to most complex
- Lewis Theory
- Valence Bond Theory
- Orbital Hybridization
- Resonance
- Molecular Orbital Theory
2
Lewis Theory of Bonding
- Only the valence electrons are involved in
bonding represent these with dots - Atoms participate in bonding so as to achieve a
noble gas electron configuration (a complete
valence shell) - Octet for 2nd period and below, duet for hydrogen
- B and Al often settle for six
- Period 3 and lower can exceed octet
- Covalent Bond shared electron pair
3
Figure 1.2 A periodic table of the common
elements seen in organic chemistry
4
So lets write some Lewis Structures CCl4 NH3 H
2O CO2 CH3Br CH4O CO2 PI3 N2H4 H2CO3 C3H8
C3H6
5
Figure 1.3 Summary The usual number of bonds of
common neutral atoms
6
Formal Charge
- Formal Charge can be assigned to each atom in a
Lewis structure. It is the charge the atom would
have if its bonding electrons were being shared
even steven. - FC the of e-s needed to balance nuclear
charge minus the of e-s owned by the atom - FC group - nonbonding e-s ½ bonding e-s