p. 566 - PowerPoint PPT Presentation
1 / 27
Title:
p. 566
Description:
His main area of research was in the Organometallic Chemistry area of Grignard reagents where he developed numerous Organometallic reagents, ... – PowerPoint PPT presentation
Number of Views:109
Avg rating:3.0/5.0
Title: p. 566
1
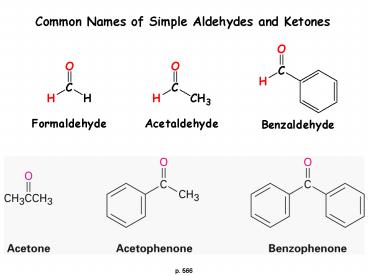
Common Names of Simple Aldehydes and Ketones
p. 566
2
Common Names of Carbonyl substituents
p. 566
3
Preparation of Aldehydes and Ketones Review
- Oxidation of 1? alcohols to aldehydes
- a. PCC
- b. Dess-Martin Periodinane
p. 567
4
Preparation of Aldehydes and Ketones Review
- Oxidation of 1? alcohols to aldehydes
- PCC
- b. Dess-Martin Periodinane
p. 567
5
Preparation of Aldehydes and Ketones Review
2. Oxidation of 2? alcohols to ketones a. CrO3 ,
H2SO4 b. K2Cr2O7, H2SO4 c. PCC d. Dess - Martin
p. 567
6
Preparation of Aldehydes and Ketones Review
2. Oxidation of 2? alcohols to ketones a. CrO3 ,
H2SO4 b. K2Cr2O7, H2SO4 c. PCC d. Dess - Martin
p. 567
7
Preparation of Aldehydes and Ketones Review
3. Aryl aldehydes and ketones Friedel Crafts
Acylation
p. 568
8
Preparation of Aldehydes and Ketones Review
3. Aryl aldehydes and ketones Friedel Crafts
Acylation
p. 568
9
Preparation of Aldehydes and Ketones New
- From Acid Chlorides via Acyl Transfer
- Using R2CuLi (Section 16.4)
p. 568
10
Preparation of Aldehydes and Ketones New
- From Acid Chlorides via Acyl Transfer
- Using R2CuLi (Section 16.4)
2. From Esters Reduction with DIBAH (Section
16.6)
p. 568
11
Oxidation of Aldehydes Review
a. CrO3, H2SO4 b. K2Cr2O7, H2SO4
p. 568
12
Oxidation of Aldehydes Review
a. CrO3, H2SO4 b. K2Cr2O7, H2SO4
p. 568
13
Nucleophilic Additions to Aldehydes and Ketones
I. Reactivity 1. Aldehydes vs. ketones 2.
Aromatic vs. aliphatic
Fig. 14-3, p. 571
14
p. 571
15
Nucleophilic Additions to Aldehydes and Ketones
I. Reactivity 1. Aldehydes vs. ketones 2.
Aromatic vs. aliphatic 3. The Burgi Dunitz
angle
Fig. 14-3, p. 571
16
Nucleophilic Additions to Aldehydes and Ketones
Fig. 14-3, p. 571
17
The Burgi-Dunitz(-Lehn-Wipff) Angle Molecular
orbital representaiton
Tetrahedron 1974 30 (12) 15631572.
18
Nucleophilic Additions to Aldehydes and Ketones
- II. Types of Nucleophiles
- 1. Anionic
- 2. Neutral
Fig. 14-3, p. 571
19
Nucleophilic Additions to Aldehydes and Ketones
- II. Types of Nucleophiles
- 1. Anionic
- 2. Neutral
- i. Water
- ii. Alcohols
- iii. 1? amines
- iv. 2? amines
Fig. 14-3, p. 571
20
Professor Gilbert Stork
Stork was born in Brussels, Belgium, and received
his secondary education in France. The B.S.
(1942) and Ph.D. (1945) were obtained at the
Universities of Florida and Wisconsin
respectively. He was on the Harvard faculty
(1946-53), then joined the Columbia faculty where
he was instrumental in building its strong
organic group and is currently Eugene Higgins
Professor Emeritus. Stork's many awards include
the ACS Award in Pure Chemistry (1957), the ACS
Award for Creative Work in Synthetic Organic
Chemistry (1967), the Arthur C. Cope Award
(1980), the Willard Gibbs Medal (1982), the
National Medal of Science (1982), the Tetrahedron
Prize (1985), the Roger Adams Award (1991) and
the Wolf Prize (1996). In 2001 he published the
first completely stereoselective total synthesis
of quinine.
http//chem.tufts.edu/AMichael-Bio.html
21
Reactions of Ylides with Aldehydes
- The Wittig Reaction
- 1. Generation and nature of ylides
- 2. The Metathesis reaction
- a. Stereochemistry
Fig. 14-3, p. 571
22
The Truth about the Wittig reaction
J. Am. Chem. Soc. 1973, 95, 5778.
23
Reactivity of a,b-unsaturated carbonly compounds
- 1. Electrophilic sites
- Hard-soft acid-base theory
- Examples
Fig. 14-3, p. 571
24
p. 589
25
Professor Author Michael
Born in Buffalo NY in 1855, he studied chemistry
at Heidelberg under Robert Bunsen (1811-1899) and
at Berlin under August Wilhelm Hofmann
(1818-1892). He then studied under Adolphe Wurtz
(1817-1884) in Paris and Dimitri Ivanoviè
Mendeleev (1834-1907) in St Petersburg, but never
bothered to take a degree. He was made Professor
of Chemistry at Tufts College near Boston. In
1889, he married one of his most brilliant
students, (1857-1904), one of the few women
organic chemists Helen Cecilia DeSilver Abbott in
this period. After a failed attempt to run the
chemistry department at Clark University in
Worcester, Massachusetts, in 1891, he spent three
years working with his wife in his private
laboratory on the Isle of Wight before returning
to Tufts. After he retired from Tufts in 1907,
Michael set up another private laboratory at
Newton Center near Boston. In 1912 he was
appointed a Professor of Chemistry, without
lecturing duties, at Harvard University. Arthur
Michael died in 1942.
http//chem.tufts.edu/AMichael-Bio.html
26
Professor Henry Gilman
(May 9, 1893 Nov 7, 1986). Born in Boston,
Massachusetts, he received his B. S. (1915), an
M. S. (1916), and a Ph.D. (1918) in Chemistry
from Harvard University. For his pre-doctoral
work he studied in Europe at Zurich
Polytechnikum, the Sorbonne in Paris, and at
Oxford in London. Dr. Gilman began his career at
the University of Illinois as an Instructor of
Chemistry in 1919. Later that year, he accepted
the position of Assistant Professor (1919 - 1920)
at Iowa State College (University). By 1923 Dr.
Gilman had been promoted to full Professor
teaching all the Organic Chemistry courses at
Iowa State College. His main area of research
was in the Organometallic Chemistry area of
Grignard reagents where he developed numerous
Organometallic reagents, including the
dialkyllithium reagent which bears his name.
http//www.lib.iastate.edu/spcl/exhibits/150/templ
ate/gilman.html
27
End