Mass spec - PowerPoint PPT Presentation
Title:
Mass spec
Description:
Lecture 5 Step Growth Chain Growth Paul Flory Clears Things Up Basic Types of Polymerization Mechanisms Chain growth system The characteristic of a ... – PowerPoint PPT presentation
Number of Views:75
Avg rating:3.0/5.0
Title: Mass spec
1
Macromolecular Chemistry
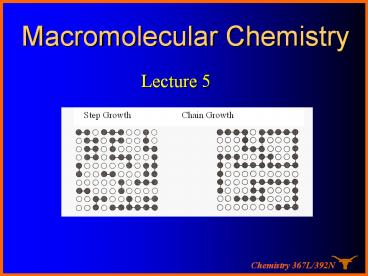
Lecture 5
Step Growth Chain Growth
2
Paul Flory Clears Things Up
Polymer Structure is distinct from polymerization
process
3
Basic Types of Polymerization Mechanisms
Step-growth
Chain-growth
Ring-opening
4
Chain growth system
- The characteristic of a chain polymer is that
polymer growth takes place by monomer reacting
only with the reactive centers. Monomer does not
react with monomer and the different-sized
species such as dimer, trimer, and n-mer do not
react with each other. The polymerization ceases
when the active center is destroyed by
termination reaction(s).
5
Step Growth system
- A condensation takes place between two
polyfunctional molecules to produce one larger
polyfunctional molecule with the possible
elimination of a small molecule such as water.
The reaction continues until one of the reagents
is used up.
6
The chain growth vs. step growth
7
- Step-growth polymerization
8
- Chain-growth polymerization
9
Chain Growth Polymerization
1
1 2 3 4 5 6
7
DP No/N 12 / 7 1.7 (for 50, b)
10
Step Growth Polymerization
DP No/N 12 / 9 1.3 (for 50, b)
11
The chain growth system
- The relationship between Mwt and conversion
- With no termination reactions
12
The chain growth system
- The relationship between Mwt and conversion
- With termination reactions
13
Step growth system
- The relationship between Mwt and conversion
14
Distinguishing features of chain- and
step-polymerization mechanisms
15
Lets look at this more closely.
Consider a flask of monomer .If there are No
molecules in the flask at time 0 and N
remaining at time t then the DP at time t is the
average degree of polymerization must just be
N0/N!
16
The Carothers Equation
If there are No molecules at time 0 and N
remaining at time t then the amount reacted is
N0-N and we can define p as the conversion or
fraction reacted then as p (No N ) / No or
N No( 1 P) If DP is the average degree of
polymerization N0/N .substituting gives
DP 1 / (1 P) and for P
0.98 (98 conversion), DP only 50!
High Molecular weights are hard to get this way
17
It all happens at the end!!!
DP 1 / (1 P)
18
A-A, B-B vs A-B ???
A .A
B..................B
A.B
19
Chain Growth Polymers
- Chain polymer growth takes place by monomer
reacting only with the reactive centers. Monomer
does not react with monomer and species such as
dimer and trimer do not react with each other.
The polymerization ceases when the active center
is destroyed by termination reactions. - Reactive intermediates in chain-growth
polymerizations include radicals, carbanions,
carbocations, and organometallic complexes
20
Radical Chain-Growth Polymers
- Look at classical example of polymerizations of
ethylene and substituted ethylenes
R
n
R
An alkene
21
Radical Chain Growth Polymerization
- Among the initiators used for radical
chain-growth polymerization are diacyl peroxides,
which decompose as shown on mild heating
22
Benzoyl peroxide is a popular pharmaceutical
23
Radical Chain Growth Polymerization
- Another common class of initiators are azo
compounds, which also decompose on mild heating
or with absorption of UV light
N
N
2
N
N
C
N
C
N
C
N
Alkyl radicals
Azoisobutyronitrile (AIBN)
24
Initiation
25
Propagation
26
Termination
Coupling
Disproportionation