Style A Square 42 PowerPoint PPT Presentation
Title: Style A Square 42
1
Correcting For Partial Volume Effects In
Perfusion MRI of Alzheimers Disease Iris
Asllani, Ajna Borogovac, Truman Brown, Christian
Habeck and Yaakov Stern Columbia University, New
York, NY 10032
Introduction
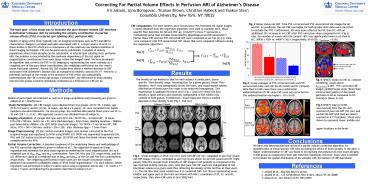
Fig. 2 below shows net CBF from PVE-corrected
and PVE-uncorrected ASL images for AD and HC. As
predicted, the net CBF was higher for both groups
when data were corrected to exclude the PVE.
Furthermore, AD were more affected by PVE than HC
with an additional 10 increase in net CBF after
PVE correction when compared to HC (Fig.2). Also,
the number of voxels with GM content gt80 was
significantly lower in AD than in HC, 36936
7024 vs. 44047 5631, respectively, (Plt0.002,
?two-tailed 0.05).
CBF computation For each subject, pure GM and
pure WM fractional ASL signal images (?M/M)
obtained from the regression algorithm were used
to compute pure, tissue specific flow densities
for GM and WM1.as (?M/M)F where F represents
a normalizing factor that includes tissue
specific physiological and MR parameters4.
Partial tissue CBF and net PVE-corrected CBF were
computed as per Eqs.1-3. Data were compared
with PVE-uncorrected CBF values that were
computed without running the regression
algorithm2.
The main goal of this study was to estimate the
pure disease-related CBF decrease in Alzheimers
Disease (AD) by excluding the atrophy
contribution via partial volume effects (PVE) in
arterial spin labeling (ASL) perfusion
MRI. Studies of aging of the brain typically rely
on imaging techniques such as PET and MRI to
characterize changes in cerebral perfusion
associated with it. A major source of error in
these studies is the PVE which are a consequence
of the relatively low spatial resolution of these
imaging techniques. PVE can be particularly
problematic in studies of elderly populations
where brain atrophy can be substantial. In
Arterial Spin Labeling (ASL) perfusion MRI, the
problem is exacerbated by the nonlinear
dependency of the ASL signal on magnetization
contributions from each tissue within the imaged
voxel. We have developed an algorithm that
corrects for PVE in ASL imaging by representing
the voxel intensity as a weighted sum of the pure
tissue contributions the weighting coefficients
are the voxels tissue fractional volumes1.
Recent ASL CBF studies have shown a marked
decrease in CBF associated with Alzheimers
Disease (AD) as compared to healthy controls
(HC)2,3. However, a potential confound of the
results is the presence of PVE which can
substantially underestimate the CBF in these age
groups. Furthermore, any difference in brain
atrophy between the groups would be mistakenly
estimated as a difference in CBF per se.
Eq.3
Results
The novelty of our method is that for each
subject it yields pure, tissue-specific flow
density maps. Assuming that for a given person,
tissue flow densities dont vary substantially
across the brain, one would expect spatial
distribution of these pure flow maps to be
relatively homogenous. This expectation is
qualitatively borne out in Fig.1 (2nd row) where
GM flow density is quite uniform and relatively
independent of the voxelss GM content in both
groups. Furthermore, visual inspection shows a
marked decrease in flow density in AD (Fig.1, 2nd
row)
Fig.4 SPMT masks for HD-AC contrast containing
voxels above puncorrectedlt0.001. PVE corrected
CBF images yielded larger areas (blue) than
uncorrected (yellow) of decreased perfusion in AD
as compared to HC. Overlap is shown in red.
Methods
Details of participant recruitement as well as of
image acquisition and processing are given in
Asllani et al.2. Briefly here Study
Participants ASL CBF images were obtained from
two groups AD (N10, 5 males, age 70.9 9.2
years), and HC (N30, 16 males, age 68.6 6
years). HC were recruited from family members and
advertisements. For the AD group, the modified
Mini Mental State score was 39.3 10 and CDR 1.2
0.42.. IRB approved consent was obtained from
all subjects. Imaging acquisition (1) Single
shot spin-echo EPI CASL TR/TE/FA 4s/36ms/90º,
15 slices, FOV220 198 mm, matrix 64 51, slice
thickness/gap 8mm/1mm, labeling duration
2000ms post-label delay 800ms. (2) 3DT1 SPGR
structural images TE/TR/FA 3 ms/34 ms/45º 100
slices, FOV 240 240 mm matrix 256 256
slice thickness/gap 1.5mm/1mm Image
Preprocessing (1) ASL control and label images
were motion corrected to the first acquired image
and registered to SPGR using SPM99. (2) SPGR was
segmented to generate GM, WM, and CSF matter
fractional volume maps. (3) SPGR and tissue
fractional volume maps were coregistered to the
EPIs. Partial Volume Correction A detailed
treatment of the underlying theory and
methodology of the PVE correction algorithm is
given in Asllani et al.1. The algorithm is based
on linear regression and estimates the pure
tissue signals by modeling the voxel
magnetization (m) as a weighted sum of MGM, MWM,
and MCSF contributions from GM, WM, and CSF,
respectively, and the ASL difference signal as a
weighted sum of ?MGM and ?MWM of the GM and WM
flow contributions, respectively. The weighting
coefficients in both cases are the tissues
fractional volume obtained from posterior
probability masks of SPGR tissue segmentation2.
For each subject, linear regression was performed
in subjects native space using a regression
kernel sise 11 voxels x 11 voxels x 1voxel, and
following the procedure described in Asllani et
al.1.
Fig.3SPMT map (plt0.001, uncorrected) from the
(HC-AD) contrast of pure GM CBF (1st row) and
partial GM CBF (2nd row) are shown overlaid on a
T1 template. Slices were shown to represent
lower, middle and upper locations in the brain.
Conclusions
We have also demonstrated the utility of a
partial volume correction algorithm for
quantification of tissue specific CBF thus
excluding the effect of brain atrophy in the
data. A higher underestimation in CBF was found
in AD implying the presence of more brain atrophy
in this group as compared to their age-matched
counterpar. However, more work is needed to
correlate the spatial distribution of the atrophy
with the pattern of CBF depression.
Fig.1 Group averages of SPGR (1st row), pure GM
CBF (2d row, computed as per Eq.1) and net CBF
image (3rd row, computed as per Eq.3) are shown
for AD (left panel) and HC (right panel). Note
the overall lower intensity of CBF images in AD
patients as compared to the age-matched elderly
controls. Also, note that pure GM CBF maps are
independent of GM fractional volume at a given
voxel thus reflecting the density of the GM flow
at that voxel, I.e., the GM flow that voxel would
have if it contained 100 GM. Slices representing
lower, middle, and upper part of the brain are
shown with MNI z-coordinate of 20, 32, and 49,
respectively. Bars show CBF units in
(mL/100gmin)
References
- Asllani et al., Mag Res Med (in press)
- Asllani et al., J of Cereb Blood Flow Met,
28(4)725-36 (2008) - 3. Alsop et al., Ann Neurol 4793-100 (2000)