Electrolysis - PowerPoint PPT Presentation
1 / 29
Title:
Electrolysis
Description:
... 11 e.g.2 Electrolysis of conc sodium chloride using mercury as cathode Slide 13 Slide 14 Slide 15 Slide 16 e.g. Extraction of Aluminium B. Purification ... – PowerPoint PPT presentation
Number of Views:1071
Avg rating:3.0/5.0
Title: Electrolysis
1
Electrolysis
2
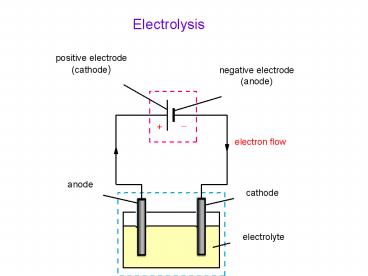
- What is electrolysis?
- By using electricity to decompose chemical
substances in which a redox reaction is forced to
take place - What is cathode and anode?
- Red cat, An ox
- What is positive and negative pole?
- According to the battery
3
A. Electrolysis of molten substance by using
inert electrode
- No preference of discharge of ions
- pole attracts negative ions, vice versa
Pb2(l) 2e- ? Pb(l)
2Br-(l) ? Br2(g) 2e-
4
B. Electrolysis of dilute solution by using inert
electrode
4OH-(aq) ? O2(g) 2H2O(l) 4e-
2H(aq) 2e- ? H2(g)
Cl- OH-
Na H
5
B. Electrolysis of dilute solution by using inert
electrode
- Preference of discharge of ions (according to the
E.C.S) - negative ions go to anode and discharge in the
order OH-gtI-gtBr-gtCl- gtgtgtgtSO42-, NO3- - e.g. OH- ions discharge , as it is more readily
to give electrons than other negative ions - positive ions go to anode and discharge in the
order AggtCu2gtH gtgtgtgtother metal ions - e.g. H ions discharge, as it is more readily to
accept electrons.( Cu2/Ag discharges if they
are present)
6
C. Electrolysis of conc solution by using inert
electrode
2Cl-(aq) ? Cl2(g) 2e-
2H(aq) 2e- ? H2(g)
7
C. Electrolysis of conc solution by using inert
electrode
- By concentration effect, the ions to be discharge
may be different from the dilution solution. - e.g. negative ions discharge according to the
order of ease to lose e-(s) OH-gtI-gtBr-gtCl- - although OH- is more readily to give electrons,
however due to concentration effect (I-/Br-/Cl-
are present in larger amount), I-/Br-/Cl- are
discharged instead. - e.g. positive ions discharge according to the
order of ease to gain e-(s) AggtCu2gtH
gtgtgtgtother metal ions - The ion discharge is same as in dilute solution
unless Mercury is used as anode.
8
D. Electrolysis of dilute solution by using
non-inert electrode
Cu2(aq) 2e- ? Cu(s)
Cu(s) ? Cu2(aq) 2e-
OH- SO4-
Cu2 H
9
The solution finally becomes
10
D. Electrolysis of dilute solution by using
non-inert electrode
- Metals ALWAYS give electrons more readily than
OH- ion. - pole metal electrode discharges, and
dissolves, but not OH- ion. - Would the preference of discharge of ions be
affected at the pole by using non-inert
electrode? - NO (metal solids always give electrons)
11
- e.g.1 Electrolysis of dil Zn(NO3)2
2H 2e- ? H2
Zn
Pt
Zn ? Zn22e-
OH- NO3-
H Zn2
dil Zn(NO3)2
12
e.g.2 Electrolysis of conc sodium chloride using
mercury as cathode
OH- Cl-
?
Na H
Reason 1) Mercury prefers to form alloy with the
metal formed. 2) conc effect
13
At the cathode (mercury)
Na(aq) e- ? Na(s) Na(s) Hg(l) ?
Na/Hg(l) sodium amalgam (alloy)
Reduction
Overall reaction at cathode 2Na/Hg(l) 2H2O(l)
? 2NaOH(aq) H2(g) 2Hg(l)
At the anode (graphite)
Oxidation
Due to concentration effect,
2Cl-(aq) ? Cl2(g) 2e-
Overall reaction 2Hg(l) 2Na(aq) 2Cl-(aq) ?
2Na/Hg(l) Cl2(g)
The sodium chloride solution becomes more and
more dilute.
14
(No Transcript)
15
FACTORS AFFECTING THE RATE OF ELECTROLYSIS
Increase the voltage
Increase the current
Speed up electrolysis
Decrease the resistance
16
Uses of electrolysis A. Extraction of some
reactive metals
Metals high in the E.C.S
Molten chlorides of metals
Aluminium
Molten oxide of aluminium
17
e.g. Extraction of Aluminium
2O2- ? O2 4e-
O2- O2-
O2- O2-
O2- O2-
O2- O2-
Al3 Al3 Al3 Al3 Al3 Al3
Al3 3e- ? Al
18
B. Purification of metals
- e.g. Purify copper metal
-
Cu(s) ? Cu2(aq) 2e-
Cu2(aq) 2e- ? Cu(s)
OH- SO42-
Cu2 H
What material is electrode made up of? What
is the assumption?
19
C. Electroplating
- e.g Electroplating of nickel
Ni(s) ? Ni2(aq) 2e-
-
Ni2(aq) 2e- ? Ni(s)
Ni2 H
OH- SO42-
Can a plastic object be electroplated?
20
Ag(s) ? Ag(aq) e-
- Criterions
- Objects to be plated always place at - pole
- The electrolyte should contain the plated metal
ions.
Ag(aq) e- ?Ag(s)
Figure 27.18 (a) A set-up for electroplating
silver on a spoon. (b) Electroplating silver on
metal cups.
Can we use this method to plate zinc on a coin?
This setting only suits for silver, copper,
nickel plating.
21
D. Production of Cl2(g), H2(g), NaOH(aq) by
electrolysis of conc. brine
Cl-
OH-
Na
H
22
At the cathode (mercury)
Na(aq) e- ? Na(s) Na(s) Hg(l) ?
Na/Hg(l) sodium amalgam (alloy)
Overall reaction at cathode 2Na/Hg(l) 2H2O(l)
? 2NaOH(aq) H2(g) 2Hg(l)
At the anode (graphite)
USEFUL CHEMICALS
Due to concentration effect,
2Cl-(aq) ? Cl2(g) 2e-
23
E. Anodizing aluminium
The resistance to corrosion of aluminium can be
enhanced by anodizing it using electrolysis. A
thicker protective layer of aluminum oxide is
formed on the surface.
-
2H(aq) 2e- ? H2
4OH- ? O2 2H2O 4e-
?
What are the advantages of anodization of
aluminium??
24
WATER POLLUTION PROBLEMS IN HONG KONG
WATER POLLUTION PROBLEMS ASSOCIATED WITH
INDUSTRIAL EFFLUENTS
Liquid wastes of electroplating
Acids
Toxic chemicals
Organic solvents
Alkalis
Metal salts
Plating sludge
27.10 Water pollution problems in Hong Kong
25
Figure 27.24 Water pollution due to industrial
effluents.
26
Figure 27.24 Water pollution due to industrial
effluents.
27
- 1) acids and alkalis e.g. H2SO4 remove oxides
NaOH removes oil - ? kill water plants and animals
- increase rusting rate of metal pipes and ships
- 2) heavy metal ions e.g. Cu2 , Ni2, Cr(VI),
Pb2 - toxic to water plants and animals
- 3) cyanide (CN -) ? very toxic
28
- 1) Reducing the volume of waste solution
- (e.g. use less water for rinsing the object)
- Recycling of the waste electrolyte.
- 3) Removal of toxic substances before disposing
- Common methods are
- a) Adding Na2CO3 to remove the acids. e.g.
- Na2CO3 H2SO4 ? Na2SO4 CO2 H2O
- b) Adding NaOH to precipitate the metal ions.
e.g. - 2NaOH(aq) NiSO4(aq) ? Na2SO4(aq) Ni(OH)2(s)
- 4) Reduce very toxic Cr(VI) compounds into less
toxic Cr(III) ions.
29
CONTROL OF CHEMICAL WASTE DISPOSAL IN HONG
KONG
Chemical waste producers such as electroplating
factories are required to register with the
Environmental Protection Department (EPD) for
proper treatment and disposal of chemical wastes.
Figure 27.26 The Chemical Waste Treatment Centre
(CWTC) at Tsing Yi Island of Hong Kong. The
Centre, having operated since May 1993, is
capable of treating a great variety of chemical
wastes.