Thompson PowerPoint PPT Presentation
Title: Thompson
1
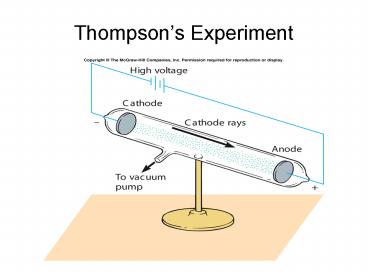
Thompsons Experiment
2
Rutherfords Experiment
3
Explanation
4
(No Transcript)
5
- Diffraction- the change in direction of a wave
as - it passes the edge of an object
6
The Wave Nature of Light
- Light as electromagnetic waves polarization,
interference, diffraction, reflection, and
refraction
7
(No Transcript)
8
Electromagnetic Spectrum
9
(No Transcript)
10
(No Transcript)
11
Atomic Line Spectra (Line Emission Spectra)
Hydrogen has the simplest atomic emission
spectrum ( 1880s).
12
The Spectrum of Atomic Hydrogen
Compare the absorption spectrum to the emission
spectrum of H
If we pass light from a continuous source (eg
from a hot object) through a substance, then the
continuous spectrum has some of the wavelengths
removed! -- absorption spectrum
13
(No Transcript)
14
Light as Particles
15
Bohr Atom
16
Hidrojen Için Bohr Atom Modeli (1913)
Elektronlar belirli yörüngelerde bulunabilirler
17
Bohr Model of Hydrogen Atoms
Assumptions
Quantized energy levels total energy for each
level is the sum of the kinetic energy of the
electron plus its potential energy. Electrons do
not radiate while in their orbits, but do when
they move from one orbit to another.
18
E? 0 eV
Paschen Series (IR)
n 3
Balmer Series (visible)
n 2
Energy
Lyman Series (ultraviolet)
E1 -13.6 eV
n 1
Lyman
Balmer
Paschen
Example Data
19
Line Spectra
The Lyman and Balmer series of lines in the
hydrogen spectrum correspond to transitions that
the electrons make between higher and lower
energy levels. The Bohr model only has one
quantum number, n, which represents the energy
level.
http//www.walter-fendt.de/ph11e/bohrh.htm
20
Electron in the Hydrogen Atom
21
- Elektronlar gerçekten de çekirdek etrafinda
belirli yörüngelerde mi dönerler? - Bunu tespit etmek mümkün mü?
22
Elektronun konumu ve momentumunun ölçülmesi
- Isinin her çarpisinda elektronun da konumu
degisir. Bu sebeple isikla bir elektronunun
konumu ve hizi hassas bir sekilde belirlenemez.
23
Heisenberg, Werner190176, Alman Fizikçi 1932
Nobel Fizik Ödülü
- Eger bir tanecigin nerede oldugunu kesin olarak
biliyorsak, ayni anda tanecigin nereden geldigini
ve nereye gittigini kesin sekilde bilemeyiz.
24
- Peki elektronlar nerede ve nasil hareket
ediyorlar?
25
Erwin Schrödinger 1927 yilinda
- Elektronlar, zamanlarinin büyük bir çogunlugunu
orbital denen bölgelerde geçirirler. - Degisik sekillerde orbitaller mevcuttur.
26
Atomik Orbitaller
- Sekli Orbital sayisi e sayisi
- s küresel 1 2
- p halter 3 6
- d karisik 5 10
- f karisik 7 14
- Herbir orbital 2 elektron içerir
27
Quantum Mechanics
28
The First Shell
- The innermost shell (1) only contains an s
orbital.
1s
29
Atomic Orbitals, s-type
30
S orbitalleri
31
Atomic Structure
32
Quantum Mechanics
33
Electron Configuration in p Orbital
34
Atomic Orbitals, p-type
35
p Orbitals
- Rather than being a sphere, the "p" orbital has
two lobes pointed in opposite direction away from
the nucleus. - One p orbital points along each the x, y, and z
axis. - There are three p orbtitals in every shell except
the first.
36
The Second Shell
- The second shell contains an s type orbital as
well as a new kind of orbital called a "p"
orbital.
2py
2px
2pz
2s
37
Atomic Orbitals, d-type
38
d Orbitals
- There are 5 types of d orbitals. Four of the five
have four lobes at 90o to one another. The fifth
looks like a donut around a p-orbital
Image from HMChem
39
The Third Shell
- The 3rd shell has
- 1 s orbital (3s)
- 3 p orbitals (3px, 3py, 3pz)
- 5 d orbitals (3dxy, 3dyz, 3dxz, 3dx2-y2, 3dz2)
40
(No Transcript)
41
f orbitals
- f-orbitals have 6 lobes and are very challenging
to envision
Image from HMChem
42
The First Two Shells
Picture from and more info on How Atoms Work
43
The Fouth Shell
- The 4th shell has
- 1 s orbital (4s)
- 3 p orbitals (4px, 4py, 4pz)
- 5 d orbitals (4dxy, 4dyz, 4dxz, 4dx2-y2, 4dz2)
- 7 f orbitals
44
Principle quantum number n 1, 2, 3,..
describes orbital size and
energy Angular momentum quantum number l 0 to
n-1 describes orbital shape
Magnetic quantum number ml l, l-1-l
describes orientation in space
of the orbital relative to the other
orbitals in the atom
Spin quantum number ms 1/2 or -1/2
describes the direction of spin of
the e- on its axis Pauli Exclusion Principle
"no two electrons in an atom can have the same
set of quantum numbers", or, only two electrons
(of opposite spin) per orbital.
45
Write a valid set of quantum numbers for each of
the following sub-shells (a) 2 s n 2, l
0, ml 0, ms - 1/2 n 2, l 0, ml 0, ms
1/2 2 combinations
46
Write a valid set of quantum numbers for each of
the following sub-shells (a) 2 s n 2, l
0, ml 0, ms - 1/2 n 2, l 0, ml 0, ms
1/2 2 combinations (b) 2 p n 2, l 1,
ml -1, ms - 1/2 n 2, l 1, ml -1, 0
or 1, ms 1/2 6 combinations
47
Write a valid set of quantum numbers for each of
the following sub-shells (a) 2 s n 2, l
0, ml 0, ms - 1/2 n 2, l 0, ml 0, ms
1/2 2 combinations (b) 2 p n 2, l 1,
ml -1, ms - 1/2 n 2, l 1, ml -1, 0
or 1, ms 1/2 6 combinations (c) 3 d n
3, l 2, ml -2, ms - 1/2 n 3, l 2, ml
-2, -1, 0, 1, or 2, ms 1/2 10 combinations
48
How many orbitals in a subshell? l 0,
1s 1 l 1, px, py, pz 3 l 2,
dxy,, dxz,, dyz ,, dx2-y2, dz2 5
49
How many orbitals in a subshell? l 0,
1s 1 l 1, px, py, pz 3 l 2,
dxy,, dxz,, dyz ,, dx2-y2, dz2 5 2 l 1
orbitals per subshell
50
How many orbitals in a subshell? l 0,
1s 1 l 1, px, py, pz 3 l 2,
dxy,, dxz,, dyz ,, dx2-y2, dz2 5 2 l 1
orbitals per subshell How many orbitals in a
shell? n 1, 1s 1 n 2, 2s, 2px, 2py,
2pz 4 n 3, 3s, 3px, 3py, 3pz, 3dxy,,
3dxz,, 3dyz ,, 3dx2-y2, 3dz2 9
51
How many orbitals in a subshell? l 0,
1s 1 l 1, px, py, pz 3 l 2,
dxy,, dxz,, dyz ,, dx2-y2, dz2 5 2 l 1
orbitals per subshell How many orbitals in a
shell? n 1, 1s 1 n 2, 2s, 2px, 2py,
2pz 4 n 3, 3s, 3px, 3py, 3pz, 3dxy,,
3dxz,, 3dyz ,, 3dx2-y2, 3dz2 9 n2 orbitals per
principal quantum level
52
- Hydrogen atom-
- all orbitals within a shell have the same energy
- electrostatic interaction between e- and proton
53
- Hydrogen atom-
- all orbitals within a shell have the same energy
- electrostatic interaction between e- and proton
- Multi-electron atoms-
- the energy level of an orbital depends not only
on the - shell but also on the subshell
- electrostatic interactions between e- and proton
and other e-
54
Orbital Energies
3dxy
3dxz
3dyz
3dx2-y2
3dz2
3px
3py
3pz
3s
Energy
2px
2py
2pz
2s
1s
55
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle
56
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle)
57
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle) 3. Try to attain maximum
number of unpaired e- spins in a given
sub-shell (Hund's Rule)
58
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle) 3. Try to attain maximum
number of unpaired e- spins in a given
sub-shell (Hund's Rule)
H (Z 1) 1s1
2s 2p
Energy
1s
59
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle) 3. Try to attain maximum
number of unpaired e- spins in a given
sub-shell (Hund's Rule)
N (Z 7) 1s2, 2s2, 2p3,
2p
2s
Energy
1s
60
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle) 3. Try to attain maximum
number of unpaired e- spins in a given
sub-shell (Hund's Rule)
B (Z 5) 1s2, 2s2, 2p1
2p
2s
Energy
1s
61
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle) 3. Try to attain maximum
number of unpaired e- spins in a given
sub-shell (Hund's Rule)
F (Z 9) 1s2, 2s2, 2p5
2p
2s
Energy
1s
62
Hydrogen 2s 3s 4s 1s
2p 3p 4p 3d 4d 4f Multi-electron
atoms 1s 2s 3s 4s 5
s 2p 3p 4p 3d 4d
63
1s 2s 2px 2py 2pz
H 1s1 He 1s2 Li 1s2, 2s1 Be 1s2,
2s2 B 1s2, 2s2, 2px1 C 1s2, 2s2, 2px1, 2py1 N
1s2, 2s2, 2px1, 2py1, 2pz1 O 1s2, 2s2,
2px2, 2py1, 2pz1 F 1s2, 2s2, 2px2, 2py2,
2pz1 Ne 1s2, 2s2, 2px2, 2py2, 2pz2
64
H 1s1 He 1s2 Li He, 2s1 Be He, 2s2
65
H 1s1 He 1s2 Li He, 2s1 Be He,
2s2 B He, 2s2, 2p1 Ne He, 2s2, 2p6 Na
He, 2s2, 2p6, 3s1 ? Ne, 3s1
66
H 1s1 He 1s2 Li He, 2s1 Be He,
2s2 B He, 2s2, 2p1 Ne He, 2s2, 2p6 Na
He, 2s2, 2p6, 3s1 ? Ne, 3s1 Mg He, 2s2,
2p6, 3s2 ? Ne, 3s2 Al Ne, 3s2, 3p1 Si
Ne, 3s2, 3p2
67
H 1s1 He 1s2 Li He, 2s1 Be He,
2s2 B He, 2s2, 2p1 Ne He, 2s2, 2p6 Na
He, 2s2, 2p6, 3s1 ? Ne, 3s1 Mg He, 2s2,
2p6, 3s2 ? Ne, 3s2 Al Ne, 3s2, 3p1 Si
Ne, 3s2, 3p2 P Ne, 3s2, 3p3 S Ne, 3s2,
3p4 Cl Ne, 3s2, 3p5 Ar Ne, 3s2, 3p6
68
- H 1s1 He 1s2
- Li He, 2s1 Be He, 2s2
- B He, 2s2, 2p1 Ne He, 2s2, 2p6
- Na He, 2s2, 2p6, 3s1 ? Ne, 3s1
- Mg He, 2s2, 2p6, 3s2 ? Ne, 3s2
- Al Ne, 3s2, 3p1 Si Ne, 3s2, 3p2
- P Ne, 3s2, 3p3 S Ne, 3s2, 3p4
- Cl Ne, 3s2, 3p5 Ar Ne, 3s2, 3p6
- outermost shell - valence shell
- most loosely held electron and are the most
important - in determining an elements properties
69
K Ar, 4s1 Ca Ar, 4s2 Sc Ar, 4s2, 3d1
Ti Ar, 4s2, 3d2
70
K Ar, 4s1 Ca Ar, 4s2 Sc Ar, 4s2, 3d1
Ca Ar, 4s2, 3d2 Zn Ar, 4s2, 3d10 Ga
Ar, 4s2, 3d10, 3p1 Kr Ar, 4s2, 3d10, 3p6
71
K Ar, 4s1 Ca Ar, 4s2 Sc Ar, 4s2, 3d1
Ca Ar, 4s2, 3d2 Zn Ar, 4s2, 3d10 Ga
Ar, 4s2, 3d10, 3p1 Kr Ar, 4s2, 3d10,
3p6 Anomalous electron configurations d5 and
d10 are lower in energy than expected Cr
Ar, 4s1, 3d5 not Ar, 4s2, 3d4
Cu Ar, 4s1, 3d10 not Ar, 4s2, 3d9
72
Electron Configuration of Ions Electrons lost
from the highest energy occupied orbital of the
donor and placed into the lowest unoccupied
orbital of the acceptor (placed according to the
Aufbau principle)
73
Electron Configuration of Ions Electrons lost
from the highest energy occupied orbital of the
donor and placed into the lowest unoccupied
orbital of the acceptor (placed according to the
Aufbau principle) Examples Na Ne,
3s1 Na Ne e- Cl Ne, 3s2, 3p5 e-
Cl- Ne, 3s2, 3p6 Mg Ne, 3s2 Mg2
Ne O He, 2s2, 2p4 O2- He, 2s2, 2p6
74
- Modern Theories of the Atom - Summary
- Wave-particle duality of light and matter
- Bohr theory
- Quantum (wave) mechanical model
- Orbital shapes and energies
- Quantum numbers
- Electronic configuration in atoms