Stereochemistry - PowerPoint PPT Presentation
1 / 56
Title:
Stereochemistry
Description:
Stereochemistry Chapter 6 Is the ... Tamiflu: a drug for influenza Transition state for action of influenza neuraminidase N-acetyl neuraminic acid O-sugar ... – PowerPoint PPT presentation
Number of Views:158
Avg rating:3.0/5.0
Title: Stereochemistry
1
Stereochemistry
Chapter 6
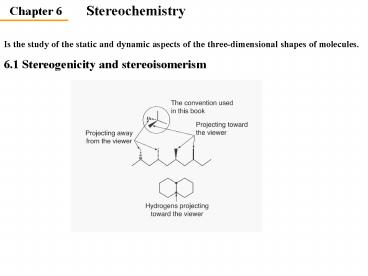
Is the study of the static and dynamic aspects of
the three-dimensional shapes of molecules.
6.1 Stereogenicity and stereoisomerism
2
6.1.1 Basic concepts and terminology
- Constitutional isomers molecules with same
molecular formular but different connectivity - between the atoms. e.g.) 1-bromo and
2-bromobutane - Stereoisomers molecules that have the same
connectivity but differ in the arrangement - of atoms in space. e.g) cis- and trans-2-butene
- 1. enantiomers nonsuperimposable mirror images
of each other - 2. diastereomers stereoisomers that are not
enantiomers - - conformational isomers are interconvertible by
rotations about single bonds - configurational isomers stereochemical isomers
including enantiomers and diastereomers. - configuration the relative position or order
of arrangement - of atoms in space which characterizes a
particular stereoisomer. - - chiral any object that is nonsuperimposable
with its mirror images - - achiral if an object is not chiral, it is
achiral.
A molecule is achiral if it is superimposable on
its mirror image. A molecule which has a plane
of symmetry, a center of symmetry or
rotation-reflection symmetry is achiral. An axis
of symmetry (C2 axis) -gt achiral? ?? ??
3
A molecule is achiral if it is superimposable on
its mirror image. A molecule which has a plane
of symmetry, a center of symmetry or
rotation-reflection symmetry is achiral. (??? ??
??)
(s, S1)
(i, S2)
meso compounds that contain stereogenic centers
but are nevertheless achiral.
4
(No Transcript)
5
Classic terminology Optically active refers to
the ability of a collection of molecules to
rotate plane polarized light - must have an
excess of one enantiomer. Racemic mixture (or
racemate) a 5050 mixture of enantiomers and is
not optically active. However, enantiomers that
do not have dramatically different refractive
indices would not result in measurable
rotations. -gt in this case, they are
optically inactive even though they are chiral.
??? optically active? ?? ???? ?? ?? ??. Chiral
center or chiral (asymmetric) carbon an atom or
specifically carbon, respectively, that has
four different ligands attached. Chiral carbons
exist in molecules that are neither asymmetric
nor chiral. Many molecules can exist in
enantiomeric forms without having a chiral
center. ? ?? ???? ?? ?? ??.
chiral center
achiral compound
6
More modern terminology Stereocenter
(stereogenic center) use this term instead of
chiral center, it is stereogenic center if the
interchange of two ligands attached to it can
produce a new stereoisomer. A
non-stereogenic center is one in which exchange
of any pair of ligands does not produce a
stereoisomer. -gt the term stereogenic center
is broader than the term chiral center.
A CWXYZ center does not guarantee a chiral
molecule. However, a CWXYZ group is always a
stereogenic center.
7
stereogenic center ??? ???? ??? stereoisomers ?
???
meso form
Typically, a molecule with n stereogenic,
tetracoordinate carbons will have 2n
stereoisomers - 2n-1 diastereomers that exist as
a pair of enantiomers.
Epimers are diastereomers that differ in
configuration at only one of the several
stereogenic centers. Carbohydrates a-
and b-anomers? epimers? ? ???.
8
6.1.2 Stereochemical descriptors
R, S system (Cahn-Ingold-Prelog system)
rectus (right) sinister (left)
higher atomic number higher priority isotopes
(the one with higher mass is assigned the higher
priority)
Tricoordinate -gt stereogenic center
9
E, Z system
higher
lower
If an H atom is on each of the double bond,
conventionally, cis and trans can be used.
Opposite E (entgegen) (cf) same Z (zusammen)
10
D, L system
mainly used for amino acids and carbohydrates
Fischer projection
Horizontal lines bonds coming out of the plane
of the paper Vertical lines bonds projecting
behind the plane of the paper
The most oxidized group top CH2OH
(carbohydrates) or R (amino acids) bottom
D dextro, right L levo, left
D
D
L
D
L
Natural amino acids L-amino acids
Important point No direct relationship between
the R/S and D/L and the sign of optical rotation
of the molecule.
11
Helical descriptors M, P system
Many chiral molecules lack a conventional center
that can be described by R/s or E/Z. -gt typically
helical, propeller, screw-shaped structures -gt a
right-handed helix (clockwise) P (plus), a left
handed helix (anti-clockwise) M (minus)
12
6.1.3 Distinguishing enantiomers
Chiral column chromatography
13
Enantiomeric excess (Xa Xb) x 100, Xa mole
fraction of a, Xb mole fraction of b High field
NMR spectroscopy with chiral shift reagents NMR
spectroscopy of derivatives that are
diastereomeric Chromatography (HPLC and GC) with
chiral stationary phases
14
NMR spectroscopy of derivatives that are
diastereomeric
(Moshers reagent)
Methods (R/S) racemate (R)-MTPA-Cl
50 50 (R-R-MTPA S-R-MTPA)
OH, NH2, SH ?
R
S
ppm
R, S peak ??
sample (R)-MTPA-Cl
Derivatives
ee 80
R
S
90 10
15
OMe
a-H
NH
L D
D
D L
D L
D
D
gt 98ee
a-H
OMe
NH
L D
D
D L
D L
D
D
gt 98ee
16
Optical activity and chirality
Optical activity the ability of a sample to
rotate a plane of polarized light. A rotation to
the right or dextrorotatory (d) A rotation to
the left - or levorotatory (l)
Optical activity establishes that a sample is
chiral, but a lack of optical activity does not
prove a lack of chirality.
17
Optical activity (a) Specific optical activity
a aD25 -gt sodium D line (589 nm emission line
of sodium arc lamp) Optical purity ()
a mixture of enantiomer
x 100
a pure enantiomer
18
6.2 Symmetry and stereochemistry
6.2.1 Basic symmetry operations
Proper rotation (Cn) -gt a rotation around an axis
by (360/n)o that has the net effect of leaving
the position of the object unchanged. C2 180
rotation, C3 120 rotation
Improper symmetry (Sn) -gt rotation and
reflection involves a rotation of (360/n)o,
combined with a reflection across a mirror plane
that is perpendicular to the rotation axis. S1
just a mirror reflection (s) S2 equivalent to
a center of inversion (i)
19
90o
60o
180o
20
6.2.2 Chirality and symmetry
A necessary and sufficient criterion for
chirality is an absence of Sn axes the
existence of any Sn axis renders an object
achiral.
C2
Asymmetric is defined as the complete absence of
symmetry. However, many chiral molecules have
one or more proper rotation axes-just no improper
axes are present. These compounds can be
referred to as dissymmetric, essential a synonym
for chiral. Thus, while all asymmetric molecules
are chiral, not all chiral molecules are
asymmetric.
21
(No Transcript)
22
6.3 Topicity relationship
Topicity derived from the same roots as
topography and topology, relating to the spatial
position of an object.
6.3.1 Homotopic, enantiotopic, and diastereotopic
Homotopic is defined as interconvertable by a Cn
axis of the molecule.
chiral influence cannot distinguish these methyl
groups
C2
23
- Heterotopic the same groups or atoms in
inequivalent constitutional or stereochemical
environment. - Enantiotopic interconverted by an Sn axis of
the molecule (n 1 in this case). - enantiotopic groups, when exposed to a chiral
influence (chiral shift reagent? ??? ?), - become distinguishable, as if they were
diastereotopic. - diastereotopic the same connectivity, but there
is no symmetry operation that - interconverts them in any conformation. ??
stereogenic center? ?? ?? - the environments of diastereotopic groups are
topologically nonequivalent. -gt they can be - distinguished by physical probes, especially
NMR spectroscopy (AB quartet)
24
2H
H1
H2
AB quartet
25
6.3.2 Topicity descriptors Pro-R/Pro-S and Re/Si
pro-S
pro-R
pro-S
pro-R
pro-R
pro-S
26
Enzymatic reactions
27
6.4 Reaction stereochemistry stereoselectivity
and stereospecificity
6.4.1 Simple guidelines for reaction
stereochemistry
28
1. Homotopic groups cannot be differentiated by
chiral reagents. 2. Enantiotopic groups can be
differentiated by chiral reagents. 3.
Diastereotopic groups are differentiated by
achiral and chiral reagents.
6.4.2 Stereospecific and stereoselective reactions
Stereospecific reaction one stereoisomer of the
reactant gives one stereoisomer of the product,
while a different stereoisomer of the reactant
gives a different stereoisomer of product.
Stereospecific reaction is a special, more
restrictive case of a stereoselective
reaction. Stereoselective reaction one in which
a single reactant can give two or more
stereoisomeric products, and one or more of these
products is preferred over the others-even if the
preference is very small. Regioselective
reaction when more than one site reacts, this
reaction is one where an excess of one of the
possible products results.
29
stereospecific
stereoselective
stereoselective
30
stereospecific
inversion
Syn addition
anti elimination
31
Regioselective reaction
Markovnikov addition
32
6.5 Symmetry and time scale
Time scale is important.
three Hs -gt equivalent due to fast rotation of
C-C bond
three Hs -gt equivalent but at low temperature
(-90 oC), inequivalent due to slow rotation (very
clowded system)
achiral lt- fast inversion
chiral lt- slow inversion
33
6.8 Stereochemical issues in chemical biology
6.8.1 The linkages of proteins, nucleic acids,
and polysaccharides
Proteins
planar
19 kcal/mol rotation barrier
4 kcal/mol preference
Much smaller cis-trans preference
34
20 natural amino acids (L form)
achiral
35
3
Nucleic acids
5
A T G ? C
5
Phosphodiester bonds
3
Nucleic acid (RNA or DNA)
36
Bases
37
b-glycosidic linkage
38
(No Transcript)
39
Phosphodiester linkages
40
Functional Glycomics
Carbohydrates
Carbohydrate-protein Interactions
Functional Glycomics
Structural and functional studies of whole
carbohydrates Studies of carbohydrate-protein
interactions
Understanding biological processes
Development of therapeutic agents
Biological processes Fertilization,
development, differentiation, growth, aging
Diseases Tumor metastasis Inflammation
Bacterial and viral infection
- - Inhibitors for carbohydrate biosynthesis
- - Inhibitors for carbohydrate-binding proteins
- Carbohydrate-based vaccines
- Finding disease-related markers
41
Glycoconjugates
Carbohydrates exist in the forms of
glycoconjugates such as glycolipids and
glycoproteins
Glycoproteins glycans attached to
proteins Glycolipids glycans attached to lipids
Cell surface carbohydrares
42
anomeric center
- Homopolysaccharides- heteropolysaccharides
Polysaccharides
- Complex carbohydrates in which many simple
sugars are linked. - Cellulose and starch are the
two most widely occurring polysaccharides in
plants.
Cellulose (-Glcb1,4Glc-)n
4
- Consists of thousands of D-glucopyranosyl-1,4-?
-glucopyranosides. - form a large aggregate
structures held together by hydrogen bonds. - is
the main component of wood and plant fiber. - is
not digested in human body but is digested in
herbivore (????).
43
Starch (?? ?? ?? ?? ??)
- is digested into glucose. - can be separated
into two fractions 1) amylose, insoluble in
cold water, 20 by weight of starch,
1,4-?-glycoside polymer 2) amylopectin,
soluble in cold water, 80 by weight of starch
contains 1,6-a-glycoside branches
approximately every 25 glucose units in addition
to 1,4-?-links.
amylose (-Glca1,4Glc-)n
Amylopectin
In human, glycosidases highly selectively
hydrolyze 1,4-?-linkage in starch but not 1,4-?
linkage in cellulose.
44
Monosaccharides in mammalian glycoconjugates
Glycosidic Bonds
45
Blood type
46
Pathogen Infection by Carbohydrate-protein
Interactions
pathogens
DNA or RNA
v Human influenza viruses (haemagglutinin
protein) preferentially adhere to NeuNAca2,6Gal
residues on epithelial cells (????) of the
lungs and upper respiratory tract. v Avian
influenza viruses (AI, ???? ????) are specific
for NeuNAca2,3Gal residues on intestinal
epithelial cells. v Some of Helicobacter pyroli
expresses Leb-binding adhesin (BabA) and sialyl
Lex-binding adhesin (SabA) and thus adhere
to the human gastric mucosa expressing these
glycans. v Cholera toxin adheres to ganglioside
GM1 in host cells.
47
Tamiflu a drug for influenza
Tamiflu (?? ???)
Transition state for action of influenza
neuraminidase
neuraminidase
O-sugar
essential for influenza virus
N-acetyl neuraminic acid
48
Stereochemical Terminology
Absolute configuration. A designation of the
position or order of arrangement of the ligands
of a stereogenic unit in reference to an agreed
upon stereochemical standard. Achiral Not
chiral. A necessary and sufficient criterion for
achirality in a rigid molecule is the presence of
any improper symmetry element (Sn including s and
?). A chirotopic. The opposite of chirotopic.
See chirotopic below. Anomers. Diastereomers
of glycosides or related cyclic forms of sugars
that are specifically epimers at the anomeric
carbon (C1 of an aldose, or C2, C3, etc., of a
ketose). Anti. Modern usage is to describe
relative configuration of two stereogenic centers
along a chain. The chain is drawn in zigazg form,
and if two substituent s are on opposite sides of
the plane of the paper, they are designated anti.
See also syn, antiperiplanar, and
anticlinal. Anticlinal. A term describing a
conformation about a single bond. In A-B-C-D, A
and D are anticlinal if the torsion angle between
them is between 90 and 150 or -90 and -150. See
Figure 2.7. Antiperiplanar. A term describing a
conformation about a single bond. In A-B-C-D, A
and D are antiperiplanar if the torsion angle
between them is between 150 to -150 . See
Figure 2.7.
49
Apical, axial, basal, and equatorial. Terms
associated with the bonds and positions of
ligands in trigonal bipyramidal structures.
Asymmetric. Lacking all symmetry elements
(pointing group C1). All asymmetric molecules are
chiral. Asymmetric carbon atom. Traditional
term used to describe a carbon with four
different ligands attached. Not recommended in
modern usage. Atactic. A term describing the
relative configuration along a polymer backbone.
In an atactic polymer, the stereochemistry is
random-no particular pattern or bias is seen.
Atropisomers. Stereoisomers ( can be either
enantiomers or diastereomers) that can be
interconverted by rotation about single bonds and
for which the barrier to rotation is large enough
that the stereoisomers can be separated and do
not interconvert readily at room temperature.
Chiral. Existing in two forms that are related
as non-congruent mirror images. A necessary and
sufficient criterion for chirality in a rigid
molecule is the absence of any improper symmetry
elements. Chiral center. Older term for a
tetracoordinate carbon or similar atom with four
different substituents. More modern, and
preferable, terminology is stereogenic center
(or stereocenter)
50
Chirotopic. The term used to denote that an atom,
point, group, face, or line resides in a chiral
environment. Cis. Describing the
stereochemical relationship between two ligands
that are on the same side of a double bond or a
ring system. For alkenes only, Z is
preferred. Configuration. The relative position
or order of the arrangement of atoms in space
that characterizes a particular
stereoisomer. Conformers or conformational
isomers. Stereoisomers that are interconverted by
rapid rotation about a single bond. Constitutiona
lly heterotopic. The same groups or atoms with
different connectivities. D and L. An older
system for identifying enantiomers, relating all
stereocenters to the sense of chirality of D- or
L-glyceraldehyde. See discussion in the text.
Generally not used anymore, except for biological
structures such as amino acids and
sugars. Diastereomers. Stereoisomers that are
not enantiomers.
51
Diastereomeric excess (de). In a reaction that
produces two diastereomeric products in amounts A
and B, de 100 (A B) / (A B).
Diastereotopic. The relationship between two
regions of a molecule that have the same
connectivity but are bit related by any kind of
symmetry operation. Dissymmetric. Lacking
improper symmetry operations. A synonym for
chiral, but not the same as asymmetric .
Eclipsed. A term describing a conformation
about a single bond. In A-B-C-D, A and D are
eclipsed if the torsion angle between them is
approximately 0. Enantiomers. Molecules that
are related as non-congruent mirror
images. Enantiomeric excess (ee). In a reaction
that produces two enantiomeric products in
amounts A and A , ee 100 (A A) / (A
A). Enantiotopic. The relationship between two
regions of a molecule that are realated only by
an improper symmetry operation, typically a
mirror plane. Endo. In a bicyclic system, a
substituent that is on a bridge is endo if it
points toward the larger of the two remaining
bridges. See also exo . Epimerization. The
interconversion of epimers.
52
Epimers. Diastereomers that have the opposite
configuration at only one of two or more
stereogenic centers. Erythro and threo.
Descriptors used to distinguish between
diastereomers of an acyclic structure having two
stereogenic centers. When placed in a Fischer
projection using the convention proper for
carbohydrates, erythro has the higher priority
groups on the same side of the Fischer
projection, and threo has them on opposite
sides. Exo. In a bicyclic system, a substituent
that is on a bridge is exo if it points toward
the smaller of the two remaining bridges. See
also endo . E, Z. stereodescriptors for
alkenes (see discussion in the text). Gauche. A
term describing a conformation about a single
bond, In A-B-C-D, A and D are gauche if the
torsion angle between them is approximately
60(or -60). See section 2.3.1. Geminal.
Attached to the same atoms. The two chlorines of
1,1-dichloro-2,2-difluoroethane are geminal. See
also vicinal. Helicity. The sense of chirality
of a helical or screw shaped entity right (P)
or left (M).
53
Heterochiral. Having an oppsite sense of
chirality. For example, D-alanine and L-leucine
are heterochiral. See also homochiral. Heteroto
pic. The same groups or atoms in inequivalent
constitutional or stereochemical
environments. Homochiral. Having the same sense
of chirality. For example, the 20 natural amino
acids are homochiral they have the same
arrangement of amino, carboxylate, and side chain
groups. Has also been used as a synonym for
enantiomerically pure, but this is not
recommended, because homochiral already as a
well-defined term before this alternative usage
became fashionable. Homotopic. The relationship
between two regions of a molecule that are
related by a proper symmetry operation.
Isotactic. A term describing the relative
configuration along a polymer backbone. In an
isotactic polymer, all stereogenic centers of the
polymer backbone have the same sense of
chirality. Meso. A term describing a achiral
member of a collection of diastereomers that also
includes at least one chiral member. Opitcally
active. Rotating plane polarized light. Formerly
used as a synonym for chiral, but this is not
reconmmended.
54
Prochiral. A group is prochiral if it contains
enantiotopic or diastereotopic ligands or faces,
such that replacement of one ligand or addition
to one face produces a stereocenter. See section
6.3.2. R, S. The designations for absolute
stereochemistry (see earlier discussion in the
text). Racemic mixture or racemate. Comprised
of a 5050 mixture of enantiomers. Relative
configuration. This refers to the configuration
of any stereogenic center with respect to another
stereogenic center. If one center in a molecule
is known as R, then other centers can be compared
to it using the descriptors R or S, indicating
the same or opposite stereochemistry,
respectively. Resolution. The separation of a
racemic mixture into its individual component
enantiomers. Scalemic. A synonym for
non-racemic or enantiomerically enriched. It
has not found general acceptance, but is used
occasionally. S-cis and s-trans. Descriptors
for the conformation about a single bond, such as
the C2-C3 bond in 1,3-buadiene, or the C-N bond
of an amide. If the substituents are
synperiplanar, they are termed s-cis (s for
single) if they are antiperiplanar, they are
termed s-trans.
55
Stereocenter. See stereogenic center. Stereogen
ic center. An atom at which interchange of any
two ligands produces a new stereoiosmer. A
synonym for stereocenter. Stereogenic unit.
An atom or grouping of atoms at which interchange
of any two ligands produces a new
stereoisomer. Stereoisomers. Molecules that have
the same connectivity, but a different
arrangement of atoms in space. Stereoselective.
A term describing the stereochemical consequences
of certain types of reactions. A stereoselective
reaction is one for which reactant A can give two
or more stereoisomeric products, B and B, and
one or more product is preferred. There can be
degrees of stereoselectivity. All stereospecific
reactions are stereoselective, but the converse
is not true. Stereospecific. A term describing
the stereochemical consequences of certain types
of reactions. A stereospecific reaction is one
for which reactant A gives product B, and
stereoisomeric reactant A gives stereoisomeric
product B. There can be degrees of
stereospecificity. Stereosprcific does not means
100 stereoselective.
56
Syn. Modern usage is to describe the relative
configuration of two stereogenic centers along a
chain. The chain is drawn in zigzag form, and if
two substituents are on the same side of the
plane of the paper, they are syn. See also
anti, synperiplanar, and synclinal. Synclin
al. A term describing a configuration about a
single bond. In A-B-C-D, A and D are synclinal if
the torsion angle between 30 and 90 (or -30
and -90). See Figure 2.7. Syndiotactic. A term
describing the relative configuration along a
polymer backbone. In a syndiotactic polymer, the
relative configuration of backbone stereogenic
centers alternate along the chain. Synperiplanar.
A term describing a conformation about a single
bond. In A-B-C-D, A and D aresynperiplanar if the
torsion angle between them is between 30 and
30. See Figure 2.7. Tacticity. A generic term
describing the stereochemistry along a polymer
backbone. See atactic, isotactic, and
syndiotactic. Trans. A term describing the
stereochemical relationship between two ligands
that are on opposite sides of a double or a ring
system. For alkenes only. E is preferred. Vicinal
. Attatched to adjacent atoms. In
1,1-dichloro-2,2-difluoroethane, the relationship
of either chlorine to either fluorine is vicinal.
See also geminal.