Stoichiometry - PowerPoint PPT Presentation
Title: Stoichiometry
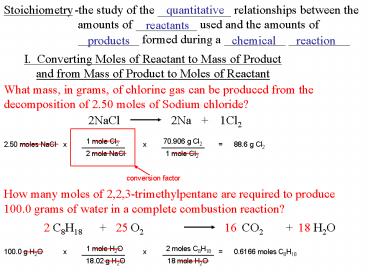
1
Stoichiometry
-the study of the ____________ relationships
between the amounts of __________ used and the
amounts of __________ formed during a __________
__________
quantitative
reactants
products
chemical
reaction
I. Converting Moles of Reactant to Mass of
Product and from Mass of Product to Moles of
Reactant
What mass, in grams, of chlorine gas can be
produced from the decomposition of 2.50 moles of
Sodium chloride?
2NaCl
2Na
1Cl2
1 mole Cl2
70.906 g Cl2
2.50 moles NaCl
x
___________
88.6 g Cl2
x
___________
2 mole NaCl
1 mole Cl2
conversion factor
How many moles of 2,2,3-trimethylpentane are
required to produce 100.0 grams of water in a
complete combustion reaction?
CO2
O2
H2O
C8H18
18
2
16
25
1 mole H2O
2 moles C8H18
100.0 g H2O
x
___________
0.6166 moles C8H18
x
_____________
18.02 g H2O
18 mole H2O
2
Stoichiometry
I. Converting Moles of Reactant to Mass of
Product and from Mass of Product to Moles of
Reactant
What mass, in grams, of citric acid (H3C6H5O7)
can be produced from the fermentation of 7.500
moles of sucrose (C12H22O11) in air?
H3C6H5O7
O2
H2O
C12H22O11
3
1
2
3
2 mole H3C6H5O7
192.12 g H3C6H5O7
7.500 moles C12H22O11
x
______________
2882 g H3C6H5O7
x
_________________
1 mole C12H22O11
1 mole H3C6H5O7
How many moles of Copper(II) sulfate are required
to produce 50.0 grams of Zinc sulfate in a
single displacement reaction?
ZnSO4
Zn
Cu
CuSO4
1
1
1
1
1 mole ZnSO4
1 mole CuSO4
50.0 g ZnSO4
x
___________
0.310 moles CuSO4
x
_____________
161.5 g ZnSO4
1 mole ZnSO4
3
Stoichiometry
II. Converting Mass of Reactant to Mass of
Product and from Mass of Product to Mass of
Reactant
What mass, in grams, of water can be obtained
from the decomposition of 25.0 grams of Ammonium
nitrate?
1NH4NO3
1N2O
2H2O
1 mole NH4NO3
2 moles H2O
18.02 grams H2O
25.0 grams NH4NO3
x
______________
11.3 g H2O
x
___________
x
_____________
80.04 grams NH4NO3
1 mole NH4NO3
1 mole H2O
conversion factor
What mass, in grams, of methane is required to
produce 50.0 grams of chloroform?
1CH4
1CHCl3
3HCl
3Cl2
1 mole CHCl3
1 mole CH4
16.043 grams CH4
50.0 grams CHCl3
x
______________
6.72 g CH4
x
___________
x
______________
119.38 grams CHCl3
1 mole CHCl3
1 mole CH4
4
Stoichiometry
II. Converting Mass of Reactant to Mass of
Product and from Mass of Product to Mass of
Reactant
What mass, in grams, of nitrogen can be obtained
from the decomposition of 100.0 grams of Sodium
azide?
2NaN3
3N2
2Na
1 mole NaN3
3 moles N2
28.014 grams N2
100.0 grams NaN3
x
______________
64.64 g N2
x
___________
x
_____________
65.011 grams NaN3
2 mole NaN3
1 mole N2
What mass, in grams, of Hydrogen is required to
produce 45.0 grams of methanol, in a synthesis
reaction with Carbon monoxide ?
1CO
1CH3OH
2H2
1 mole CH3OH
2 mole H2
2.016 grams H2
45.0 grams CH3OH
x
______________
5.66 g H2
x
___________
x
______________
32.042 grams CH3OH
1 mole CH3OH
1 mole H2
5
Stoichiometry
III. Determining the Limiting Reactant
If 200.0 grams of sulfur react with 100.0 grams
of chlorine in a synthesis reaction, what mass,
in grams, of Disulfur dichloride is produced?
1S8
4Cl2
4S2Cl2
1 mole S8
200.0 g S8
x
___________
0.7796 moles S8
_____________
0.7796
In excess
256.53 g S8
1 mole S8
1 mole Cl2
100.0 g Cl2
x
___________
1.410 moles Cl2
_____________
0.3525
Limiting reactant
70.906 g Cl2
4 moles Cl2
1 mole Cl2
4 moles S2Cl2
135.038 grams S2Cl2
100.0 grams Cl2
x
______________
190.4 g S2Cl2
x
___________
x
_______________
70.906 grams Cl2
4 moles Cl2
1 mole S2Cl2
6
Stoichiometry
III. Determining the Limiting Reactant
If 25.0 grams of phosphorus react with 50.0 grams
of oxygen in a synthesis reaction, what mass, in
grams, of Tetraphosphorus decoxide is produced?
1P4
5O2
1P4O10
1 mole P4
25.0 g P4
x
___________
0.202 moles P4
_____________
0.202
Limiting reactant
123.90 g P4
1 mole P4
1 mole O2
50.0 g O2
x
___________
1.56 moles O2
_____________
0.312
In excess
31.998 g O2
5 moles O2
1 mole P4
1 mole P4O10
283.89 grams P4O10
25.0 grams P4
x
______________
57.3 g P4O10
x
___________
x
_______________
123.90 grams P4
1 mole P4
1 mole P4O10
7
Stoichiometry
IV. Calculating Percent Yield
-the ________ ______ is the ______ of the
_______ ______ to the ____________ ______
expressed as a ________
percent
yield
ratio
actual
yield
theoretical
yield
Actual Yield
_____________
Percent Yield
x
100
Theoretical Yield
percent
If 0.500 grams of Silver nitrate react with 0.500
grams of Potassium chromate and 0.455 grams of
Silver chromate is produced, what is the percent
yield of Silver chromate?
2AgNO3(aq)
1K2CrO4(aq)
1Ag2CrO4(s)
2KNO3(aq)
1 mole AgNO3
0.500 g AgNO3
x
___________
0.00294 moles AgNO3
_________________
0.00147
Limiting reactant
169.87 g AgNO3
2 moles AgNO3
1 mole K2CrO4
0.500 g K2CrO4
x
___________
0.00257 moles K2CrO4
_________________
0.00257
In excess
194.19 g K2CrO4
1 moles K2CrO4
1 mole AgNO3
1 mole Ag2CrO4
331.728 grams Ag2CrO4
x
______________
0.488 g Ag2CrO4
x
___________
x
__________________
0.500 g AgNO3
169.87 grams AgNO3
2 mole AgNO3
1 mole Ag2CrO4
Theoretical Yield
Actual Yield
0.455 g Ag2CrO4
_____________
_____________
Percent Yield
x
100
Percent Yield
x
100
93.2
Theoretical Yield
0.488 g Ag2CrO4
8
Stoichiometry
IV. Calculating Percent Yield
If 40.0 grams of Hydrogen fluoride react with
40.0 grams of Silicon dioxide and 45.8 grams of
Dihydrogen hexafluorosilicate is produced, what
is the percent yield of Dihydrogen
hexafluorosilicate?
1SiO2(s)
6HF(aq)
1H2SiF6(aq)
2H2O(l)
1 mole HF
40.0 g HF
x
___________
2.00 moles HF
___________
0.333
Limiting reactant
20.006 g HF
6 moles HF
1 mole SiO2
40.0 g SiO2
x
___________
0.666 moles SiO2
_________________
0.666
In excess
60.084 g SiO2
1 moles SiO2
1 mole HF
1 mole H2SiF6
144.09 grams H2SiF6
x
__________
48.0 g H2SiF6
x
___________
x
__________________
40.0 g HF
20.006 g HF
6 mole HF
1 mole H2SiF6
Theoretical Yield
Actual Yield
45.8 g H2SiF6
_____________
_____________
Percent Yield
x
100
Percent Yield
x
100
95.4
Theoretical Yield
48.0 g H2SiF6