Assessment of Mutant Homozygosity in Gastrointestinal PowerPoint PPT Presentation
1 / 1
Title: Assessment of Mutant Homozygosity in Gastrointestinal
1
Assessment of Mutant Homozygosity in
Gastrointestinal Stromal Tumors Michelle
Wallander1, Carlynn Willmore-Payne1 and Lester
Layfield1,2 1ARUP Institute for Clinical and
Experimental Pathology, ARUP Laboratories, Salt
Lake City, UT 2Department of Pathology,
University of Utah, Salt Lake City, UT
Introduction
Results
Activated kinases such as EGFR, BRAF and C-KIT
are associated with multiple human malignancies.
Gain-of-function deletions, insertions or point
mutations cause constitutive kinase activation,
which stimulates cell proliferation and survival.
Given the presumed dominance of the mutation, it
is not surprising that the majority of activating
mutations are heterozygous. However, homozygous
C-KIT and BRAF mutations have been reported in
melanoma. The zygosity of kinase activating
mutations may have important implications for
oncogenesis or treatment response. Mutant
zygosity has not been extensively studied in
gastrointestinal stromal tumors (GISTs), which
are characterized by activating mutations in the
receptor tyrosine kinases C-KIT or PDGFRA. We
reviewed a series of mutant GISTs, which were
previously analyzed by high resolution melting
analysis (HRMA) and DNA sequencing. The
percentage of mutant homozygosity in C-KIT exons
9, 11, 13, 17 and PDGFRA exons 12 and 18 was
determined. Mutant homozygosity was also
correlated with tumor location, size and C-KIT
(CD117) immunohistochemistry.
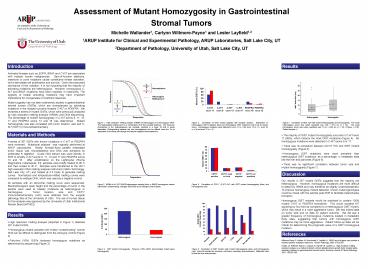
Figure 1. High resolution melting analysis
(HRMA) of heterozygous and homozygous DNA. The
heterozygote melting curve is a combination of
all four possible duplexes. The base pair
mismatches in the heteroduplexes are unstable,
resulting in a lower Tm that is easily
detectable. Distinguishing between the two
homoduplexes can be difficult since the Tm is
dependent on the base pair change and nearest
neighbor thermodynamics.
Figure 4. Correlation of GIST mutant zygosity
with mutation location. Distribution of
heterozygous GIST mutations (blue) and homozygous
GIST mutations (red) in all six tested exons.
Homozygous mutations were detected in exon 11 (n
36), exon 13 (n 1), exon 18 (n 3) and exon
12 (n 2).
Figure 7. Correlation of GIST size with mutant
heterozygosity and homozygosity. The mean
heterozygous tumor size (when specified) was 7.84
? 6.73 cm (n 34). The mean homozygous tumor
size (when specified) was 11.52 ? 9.63 cm (n
16). P-Value 0.181. Outlier samples .
Materials and Methods
- The majority of GIST mutant homozygosity
occurred in C-KIT exon 11 (86), which harbors
the most GIST mutations (Figure 4). No
homozygous mutations were detected in C-KIT exons
9 or 17. - There was no correlation between CD117 IHC and
GIST mutant homozygosity (Figure 5). - Homozygous GIST mutations were more prevalent
than heterozygous GIST mutations, as a
percentage, in metastatic sites like the liver
and pancreas (Figure 6). - There was no significant correlation between
tumor size and mutant homozygosity (Figure 7).
A series of 267 GISTs with known mutations in
C-KIT or PDGFRA were reviewed. Mutational
analysis was originally performed at ARUP
Laboratories. Briefly, formalin-fixed paraffin
embedded tumor tissue was microdissected and DNA
was extracted by proteinase K digestion. Crude
DNA extract was used directly in PCR to amplify
C-KIT exons 9, 11, 13 and 17 and PDGFRA exons 12
and 18. After amplification on the LightCycler
(Roche Diagnostics, Indianapolis, IN), samples
were briefly heated to 95C and then cooled to
40C. Samples were transferred to the HR-1
high-resolution DNA melting analysis instrument
(Idaho Technology, Salt Lake City, UT) and heated
at 0.3C/sec to generate melting curves.
Normalized and temperature-shifted melting curves
were compared to wildtype DNA, which was used as
a negative control. All samples with an abnormal
melting curve were sequenced. Electropherogram
peak height and the percentage of tumor in the
sample were used to classify mutations as
heterozygous or homozygous. Tumor location, size
and CD117 immunohistochemistry (IHC) were
obtained from the surgical pathology files at the
University of Utah. The use of human tissue for
this analysis was approved by the University of
Utah Institutional Review Board (11903).
Discussion
Our results in 267 mutant GISTs suggests that the
majority are heterozygous. However, homozygous
mutations are more difficult to detect by HRMA
and may therefore be slightly underrepresented.
To improve homozygous mutant detection, known
mutant genotypes could be mixed with the sample
post-PCR to facilitate heteroduplex
formation. Homozygous GIST mutants would be
predicted to contain 100 mutant C-KIT or PDGFRA
homodimer. This would increase KIT signaling by
four-fold as compared to a heterozygous GIST
mutant, which may result in a more aggressive
tumor. We had limited data on tumor size and no
data on patient outcome. We did see a greater
frequency of homozygous mutations located in
metastatic body sites, suggesting that tumors
with homozygous GIST mutations may be more
aggressive. Patient follow-up data will be
critical for determining the prognostic value of
a GIST homozygous mutation.
Figure 2. HRMA of a C-KIT K642E heterozygous
sample (blue), a K642E homozygous sample with
limited contaminating wild-type DNA (red) and a
wild-type control (black).
Figure 5. Correlation of CD117 (C-KIT) IHC with
GIST mutant heterozygosity (blue) and
homozygosity (red).
Results
- High resolution melting analysis (depicted in
Figure 1) detected 267 mutant GISTs. - Homozygous mutant samples with limited
contaminating normal DNA can be difficult to
distinguish from the wild-type control (Figure
2). - Forty-two (16) GISTs harbored homozygous
mutations as determined by sequencing (Figure 3).
References
Willmore-Payne C, Holden JA, Hirschowitz S,
Layfield LJ. BRAF and c-kit gene copy number in
mutation-positive malignant melanoma. Human
Pathology, 2006 37520-527. Holden JA,
Willmore-Payne C, Coppola D, Garrett CR, Layfield
LJ. High-resolution melting amplicon analysis as
a method to detect c-kit and platelet-derived
growth factor receptor alpha activating mutations
in gastrointestinal stromal tumors. American
Journal of Clinical Pathology, 2007 128230-238.
Figure 6. Correlation of GIST location with
mutant heterozygosity (blue) and homozygosity
(red). Extra-GI includes the omentum, soft
tissue, mesentary and peritoneum. Metastatic
sites include the liver and pancreas.
Figure 3. GIST mutant homozygosity. Forty-two
(16) GISTs demonstrated mutant gene homozygosity.