MSE 5310: Modeling Materials - PowerPoint PPT Presentation
1 / 41
Title:
MSE 5310: Modeling Materials
Description:
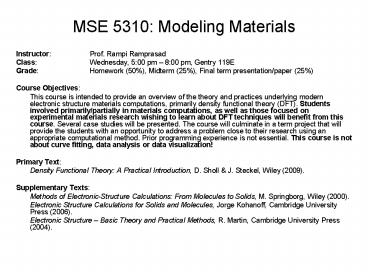
MSE 5310: Modeling Materials Instructor: Prof. Rampi Ramprasad Class: Wednesday, 5:00 pm 8:00 pm, Gentry 119E Grade: Homework (50%), Midterm (25%), Final term ... – PowerPoint PPT presentation
Number of Views:282
Avg rating:3.0/5.0
Title: MSE 5310: Modeling Materials
1
MSE 5310 Modeling Materials
- Instructor Prof. Rampi Ramprasad
- Class Wednesday, 500 pm 800 pm, Gentry 119E
- Grade Homework (50), Midterm (25), Final term
presentation/paper (25) - Course Objectives
- This course is intended to provide an overview
of the theory and practices underlying modern
electronic structure materials computations,
primarily density functional theory (DFT).
Students involved primarily/partially in
materials computations, as well as those focused
on experimental materials research wishing to
learn about DFT techniques will benefit from this
course. Several case studies will be presented.
The course will culminate in a term project that
will provide the students with an opportunity to
address a problem close to their research using
an appropriate computational method. Prior
programming experience is not essential. This
course is not about curve fitting, data analysis
or data visualization! - Primary Text
- Density Functional Theory A Practical
Introduction, D. Sholl J. Steckel, Wiley
(2009). - Supplementary Texts
- Methods of Electronic-Structure Calculations
From Molecules to Solids, M. Springborg, Wiley
(2000). - Electronic Structure Calculations for Solids and
Molecules, Jorge Kohanoff, Cambridge University
Press (2006). - Electronic Structure Basic Theory and
Practical Methods, R. Martin, Cambridge
University Press (2004).
2
Planned Topics
- Introduction the course in a nutshell (Chap. 1)
- Theory From Quantum Mechanics to Density
Functional Theory (Chap. 1) - Density Functional Theory (DFT) nuts bolts
(Chap. 3) - Reciprocal space total energy formalism
- Approximations k-point sampling,
pseudopotentials, exchange-correlation - Simple molecules solids
- Structure (Chap. 2)
- Geometry optimization (Chap. 3)
- Vibrations (Chap. 5)
- Electronic structure (Chap. 8)
- Surface science
- Periodic boundary conditions, relaxation,
reconstruction, surface energy (Chap. 4) - Chemisorption and reaction on surfaces (Chap. 4,
5, 6) - Chemical processes transition state theory
(Chap. 6) - Non-zero temperatures Thermodynamics
- Phase diagrams (Chap. 7)
- Thermal properties specific heat, thermal
expansion - Response functions elastic, dielectric,
piezoelectric constants - Beyond standard DFT
3
Lecture Topics
- Introductory comments
- Overview of theory Total energy methods and
density functional theory (DFT) - Predictions of known properties using DFT (i.e.,
validation) - Practical value of DFT calculations Insights,
and design of new materials (i.e., success
stories)
4
The need for computational science
- Anytime the future has to be predicted or
forecasted, simulation is used, generally based
on well understood scientific notions/principles - Anytime experimental analysis is too expensive or
too impractical, simulation becomes necessary - Simulations complement experiments and could
provide insights - Examples from everyday contemporary experience
- Weather modeling involves solution of Newtons
equations of motion and fluid dynamics - Astrophysical predictions (eclipses, comets)
involve solution of Newtons equations of motion
and/or general relativity - Other notable examples economic (stock market)
modeling, drug design, mechanical properties
(auto industry), electromagnetic simulations
(microelectronics industry) - Challenges
- Models by themselves may not be representative of
the real situation - Practical treatment of model (or numerical
solution) is time intensive - Sometimes the physical principles (or theory)
involved are not well known - Unknown extraneous factors, e.g., stock market
- Major numerical problems non-linear systems,
e,g, chaotic pendulum, weather - Fortunately, in Computational Materials Science
(CMS), we need to worry mainly about the first
two challenges, and the others are listed in
decreasing order of importance
5
Theory, Models, Simulation Experiments
- Theory experiment go hand in hand.
- A set of results may come out of experiment, but
one needs a theory to put it all in a framework
of understanding. A theory cannot be formulated
in the absence of experimental data - Goal of science construct theory based on
available experimental data, make predictions
using theory outside the regime of experimental
input, and modify theory if predictions are not
satisfactory - A model is a representation of physical reality,
along with a set of assumed equations that govern
that reality - Simulation is the process of using the model
using numerical techniques - CMS involves theory, modeling and simulation,
with the terminologies generally used
interchangeably!
6
Theory, Models, Simulation Experiments Example
- Let us consider an example tensile testing
- In the elastic region, we know that there is a
linear relationship between stress and strain,
which is at the heart of elasticity theory
merely having experimental data on stress versus
strain for a few materials does not constitute
true understanding the experimental data
together with the realization that stress
constantstrain constitutes understanding - Now, one can do two types of computations (1)
use the constant obtained from experimental data
to look at complicated geometries (FEM, used
widely in the auto industry), or (2) we can use a
more fundamental theory to determine the constant
from first principles the second approach
results in a even more fundamental understanding
of the origin of the constant, namely, in terms
of atomic level bond stretching - CMS sometimes complements experimental studies,
and sometimes provides insights, and increasingly
is being used to design materials
7
CMS at different scales
Time
Engineering Design
Years
Hours
Finite element Analysis (Continuum/classical)
Minutes
Seconds
Mesoscale Modeling (Semi-classical)
Microseconds
Molecular Mechanics
Nanoseconds
Quantum Mechanics
Picoseconds
Femtoseconds
mm
Å
nm
mm
m
Distance
e.g., density functional theory (DFT)
- If more than one box is involved in a
computation ? multi-scale modeling
8
Overview of CMS Course contd.
- Central themes
- Our system is represented as a collection of
atoms, or a collection of electrons and ions - We can determine the total potential energy of
this collection of particles - Equilibrium (stable, unstable and metastable)
situations correspond to features (minima,
maxima, saddlepoint) in the total potential
energy function - Analogous approaches in other types of
computations - Electromagnetic simulations involve minimization
of electromagnetic energy density - Mechanical simulations involve minimization of
strain energy - Let us for a moment assume that we do have a
prescription for computing the total energy of a
group of atoms, given their spatial positions - What kind of properties can we compute? And how?
9
Diatomic molecule, A-B
- Only one degree of freedom ? RAB
- If we new E(RAB), then we can determine potential
energy surface (PES)
Thus, IF E(RAB) is known, then we can trivially
determine equilibrium bond length, bond energy
and vibrational frequency!
10
Triatomic system, A-B-C
- Consider the reaction A-B C ? A B-C
- Two degrees of freedom in 1-dimensional A-B-C
system ? RAB, RBC - If we new E(RAB, RBC), then we can determine
potential energy surface (PES)
11
Bulk cubic material
- Only one degree of freedom ? lattice parameter a,
or Volume V ( a3) - If we new E(V), then we can determine potential
energy surface (PES)
Curvature B/V0 B bulk modulus Note slope
stress
Cohesive energy
V0
Thus, IF E(V) is known equation of state, then
we can trivially determine equilibrium lattice
parameter, cohesive energy and bulk modulus!
12
Lecture Topics
- Introductory comments
- Overview of theory Total energy methods and
density functional theory (DFT) - Predictions of known properties using DFT (i.e.,
validation) - Practical value of DFT calculations Insights,
and design of new materials (i.e., success
stories)
13
Prescriptions for computing energy
- Until 1950s, no reliable prescription was
available to practically compute the energy - breakthrough quantum mechanics, 1920s Wigner
Seitz, 1930s more later - Hence energy as a function of geometry was
parameterized using experimental data then, and
even to-date! - Lennard-Jones, Morse, etc. (physicists),
embedded-atom method, etc. (materials
scientists), force fields (chemists) - Referred to as empirical or semi-empirical
methods (as experimental data was used entirely
or partially) - Today, reliable parameter-free methods are
available to compute energy, which come with a
(rapidly diminishing) price tag of large
computational time - Density functional theory (DFT), and higher level
quantum mechanics based methods
14
Empirical approach example
- Suppose that our system contains M atoms, and
that atoms interact pairwise
summation over i and j run over the number of
atoms M
- Lennard-Jones
- Morse
- By fitting A and B (Lennard-Jones), or V0, d and
r0 (Morse) to experimental data so that
equilibrium bulk properties are reproduced, we
can in principle have a scheme to compute E - We could make the scheme more sophisticated by
defining E in terms of 3- or many-atom
interactions (e.g., embedded atom method) or
angular (e.g., Stillinger-Weber)
15
The quantum mechanical prescription
- Building blocks are N electrons and M nuclei,
rather than M atoms - The N-electron, M-nuclei Schrodinger (eigenvalue)
equation
The N-electron, M-nuclei wave function
The total energy that we seek
The N-electron, M-nuclei Hamiltonian
Nuclear kinetic energy
Electronic kinetic energy
Nuclear-nuclear repulsion
Electron-electron repulsion
Electron-nuclear attraction
- The problem is completely parameter-free, but
formidable! Why? - Cannot be solved analytically when NM gt 3
(really?!?) - Too many variables (for a 100 atom Pt cluster,
the wave function is a function of 23,000
variables!!!)
16
Formidable ? Manageable!
Density Functional Theory (DFT) W. Kohn,
Chemistry Nobel Prize, 1999
1-electron wave function (function of 3
variables!)
1-electron energy (band structure energy)
The average potential seen by electron i
Energy can be obtained from r(r), or from ?i and
ei (i labels electrons)
- Still parameter-free, but has a few acceptable
approximations - DFT is versatile in principle, it can be used to
study any atom, molecule, liquid, or solid
(metals, semiconductors, insulators, polymers,
etc.), at any level of dimensionality (0-d, 1-d,
2-d and 3-d)
17
Lecture Topics
- Introductory comments
- Overview of theory Total energy methods and
density functional theory (DFT) - Predictions of known properties using DFT (i.e.,
validation) - Practical value of DFT calculations Insights,
and design of new materials (i.e., success
stories)
18
The first convincing DFT calculationYin and
Cohen, PRB 26, 5668 (1982)
Slope is transition pressure
- The correct equilibrium phase (diamond cubic) is
predicted - The lattice parameter of the equilibrium phase,
and the pressure for the diamond cubic ? beta-tin
phase transition (common tangent) are also
predicted to a good level of accuracy
19
Predictions of geometry
- Structural details predicted typically to within
1 of experiments for a wide variety of solids
and molecules - Results from various sources collected in
Ramprasad, Shi and Tang, in Physics and Chemistry
of Nanodielectrics, Dielectric Polymer
Nanocomposites (Springer)
20
Predictions of other basic properties
21
Band offsets
22
Polarization in Periodic SystemsThe Fundamental
Difficulty
- Textbook definition
- Polarization dipole moment per unit cell volume
- inadequate depends on how unit cell is defined
unless we are in the Clausius-Mossotti limit
Dipole per unit cell well defined
Each unit cell will give a different net dipole
Resolution provided by Resta and King-Smith
Vanderbilt, in terms of phases of the
wavefunctions (Berrys phase)
23
(No Transcript)
24
Phase transformations involving solids
Experiments
Tin
Boron Nitride
Kern et al, PRB 59, 8551 (1999)
Pavone et al, PRB 57, 10421 (1998)
25
Phase transformations involving melting
expt.
expt.
DFT
DFT
26
Most-cited papers in APS journals
- Six out of the top eleven most-cited papers are
DFT-foundational papers!
27
Lecture Topics
- Introductory comments
- Overview of theory Total energy methods and
density functional theory (DFT) - Predictions of known properties using DFT (i.e.,
validation) - Practical value of DFT calculations Insights,
and design of new materials (i.e., success
stories)
28
Extreme pressures
- Extreme geophysical pressures may be difficult to
create in the lab, but can be simulated easily
29
Extreme pressures contd.
Liquid
Solid
30
Extreme pressures contd.
31
Thermal expansion
- Can a material contract when heated?
32
Si reconstruction
- When heated to high temperatures in ultra high
vacuum the surface atoms of the Si (111) surface
rearrange to form the 7x7 reconstructed surface
33
Bi makes Cu-Cu bonds softer (hence, brittleness
NOT due to electronic effects)
Grain boundary decohesion due to larger size of
Bi atoms
34
(No Transcript)
35
(No Transcript)
36
(No Transcript)
37
- High activity of transition metals in oxidation
catalysis is due to the presence of surface
oxides under catalytic conditions
38
(No Transcript)
39
What can DFT (not) do?
- Systems that can be represented in terms of up to
a few 100 atoms per repeating unit cell are okay - Geometric details to within 1 of experiments
- Other properties to within 5 of experiments
- Challenges
- Investigations requiring a large number of atoms
- Systems in which periodicity is absent
- Band gaps and excited state energies
- Non-zero and high temperatures
- Highly correlated-electron systems
40
Lecture Topics
- Introductory comments
- Overview of theory Total energy methods and
density functional theory (DFT) - Predictions of known properties using DFT (i.e.,
validation) - Practical value of DFT calculations Insights,
and design of new materials (i.e., success
stories)
41
Planned Topics
- Introduction the course in a nutshell (Chap. 1)
- Theory From Quantum Mechanics to Density
Functional Theory (Chap. 1) - Density Functional Theory (DFT) nuts bolts
(Chap. 3) - Reciprocal space total energy formalism
- Approximations k-point sampling,
pseudopotentials, exchange-correlation - Simple molecules solids
- Structure (Chap. 2)
- Geometry optimization (Chap. 3)
- Vibrations (Chap. 5)
- Electronic structure (Chap. 8)
- Surface science
- Periodic boundary conditions, relaxation,
reconstruction, surface energy (Chap. 4) - Chemisorption and reaction on surfaces (Chap. 4,
5, 6) - Chemical processes transition state theory
(Chap. 6) - Non-zero temperatures Thermodynamics
- Phase diagrams (Chap. 7)
- Thermal properties specific heat, thermal
expansion - Response functions elastic, dielectric,
piezoelectric constants - Beyond standard DFT