Radiation (Ch 12 YAC) PowerPoint PPT Presentation
1 / 9
Title: Radiation (Ch 12 YAC)
1
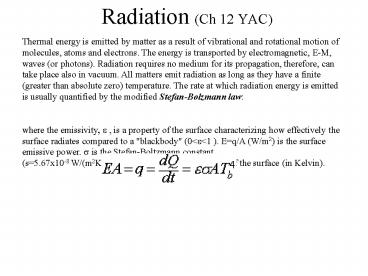
Radiation (Ch 12 YAC)
Thermal energy is emitted by matter as a result
of vibrational and rotational motion of
molecules, atoms and electrons. The energy is
transported by electromagnetic, E-M, waves (or
photons). Radiation requires no medium for its
propagation, therefore, can take place also in
vacuum. All matters emit radiation as long as
they have a finite (greater than absolute zero)
temperature. The rate at which radiation energy
is emitted is usually quantified by the modified
Stefan-Bolzmann law where the emissivity, e ,
is a property of the surface characterizing how
effectively the surface radiates compared to a
"blackbody" (0ltelt1 ). Eq/A (W/m2) is the surface
emissive power. s is the Stefan-Boltzmann
constant (s5.67x10-8 W/(m2K4)). Tb is the
absolute temperature of the surface (in Kelvin).
2
Radiation (cont.)
Blackbody is an ideal surface which emits the
maximum possible thermal radiation at a given
temperature. Irradiation (G) All radiation that
is incident on the surface. This radiation can
then be transmitted, absorbed or reflected from
the surface. G Gtrans Gabs Gref t G aG
rG Where t, a and r represent transmissivity,
absorptivity and reflectivity, respectively. t
a r 1
3
Radiation (cont. 2)
GaaG Absorbed irradiation
4
Radiation (cont. 3)
Electromagnetic Radiation Spectrum Thermal
radiation spectrum range 0.1 to 100 mm It
includes some ultraviolet (UV) radiation and all
visible (0.4-0.76 mm) and infrared radiation (IR).
5
Radiation (cont. 4)
Question Can microwave-oven heating process be
considered one kind of heat transfer
mode? Strictly speaking, it is not. It heats up
food through microwave radiation, not thermal
radiation. However, when microwave energy (with
very high frequency) interacts with water
molecules inside the food, it generates heat
inside the food. Therefore, it can be modeled as
some forms of heat generation but not relate to
any modes of heat transfer.
6
Radiation (cont. 4)
The Planck Distribution The Planck law describes
theoretical spectral distribution for the
emissive power of a black body. It can be written
as where C13.742x108 (W.mm4/m2) and
C21.439x104 (mm.K) are two constants. The planck
distribution is shown in the following figure as
a function of wavelength for different body
temperatures.
7
Spectral blackbody emissive power
8
Planck Distribution
- Points to note about Planck Distribution
- At given wavelength, the emissive power increases
with increasing temperature - As the temperature increases,more emissive
energy appear at shorter wavelengths - For low temperature (gt800 K), all radiant energy
falls in the infrared region and is not visible
to the human eyes. That is why only very high
temperature objects, such as molten iron, can
glow. - Sun can be approximated as a blackbody at 5800 K
9
Solar Irradiation