Cellular Respiration PowerPoint PPT Presentation
Title: Cellular Respiration
1
Cellular Respiration
- Giving you the energy you need!
2
Clothespin Challenge!
- Use your dominant hand
- Open and close the pin (with your thumb and
forefinger) as many times as you can for 20
seconds while holding the other fingers straight
out! - Repeat for 5 more continuous trials!
- Repeat for the non-dominant hand
3
Clothespin Challenge!
- What happened as time went on?
- How did you hands feel at the end?
- Was there a difference in dom and non-dom hands?
- Why will your muscles recover in about 10 min?
4
METABOLISM BASICS
5
1st Law of Thermodynamics
- The total amount of energy in the universe is
constant! - Energy cannot be created or destroyed but only
converted to one form into another! - Activation Energy Amount of E required to break
chemical bonds
6
2nd Law of Thermodynamics
- Entropy Randomness and Chaos
- Universe favours Entropy think of how messy
your room gets! - In all Rxns Energy and Entropy are needed!
- Spontaneous Human Combustion?
7
ENDERGONIC REACTIONS
- Energy of products more than reactants
- Photosynthesis
- Light energy converted to stored chemical energy
C6H12O6 - Every molecule of glucose contains 2870kJ
8
- Photosynthesis
9
EXERGONIC REACTIONS
- Energy of products is less than reactants
- Free energy is released!
- Cellular respiration
- The energy from glucose is released and harnessed
into ATP at a controlled rate!
10
- Cellular Respiration
11
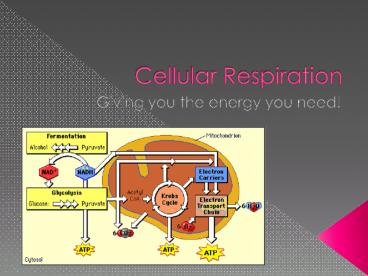
Cellular Respiration Overview
- The Goal of C.R. is to create ATP from Glucose!
- See handout!
- Four main parts...
- Glycolysis
- Pyruvate Oxidation
- Krebs Cycle (Citric Acid Cycle)
- Electron Transport Chain
12
(No Transcript)
13
Important Reactions!
- Redox Reactions
- Substrate Level Phosphorylation
- Oxidative Phosphorylation
- These rxns occur frequently throughout the
cellular respiration pathways!
14
Redox Reactions
- Energy metabolism in cells involves oxidation
reactions. - Oxidation involves the transfer of an electron
from a molecule, which is said to be oxidized, to
another molecule, which is said to be reduced. - An oxidation cannot occur without a corresponding
reduction. They are PAIRED reactions. - Many important redox reactions in cells require
the presence of coenzymes. - The redox reactions of cellular respiration
commonly involve the following coenzymes
15
Redox Rxns Make Energy Carriers
- 1) NAD Nicotinamide adenine dinucleotide
- NAD 2 e- 2 H ? NADH H
- the second H dissolves into cytosol
- 2) FAD Flavin adenine dinucleotide
- FAD 2e- 2 H ? FADH2
16
A Memory Trick!
- LEO the lion says GER
- Lose
- Electrons
- Oxidized!
- SAYS...
- Gain
- Electrons
- Reduced!
17
- Reduced means that the overall positive charge
of the molecule has decreased (due to accepting
the electons!)
18
Substrate Level Phosphorylation
- A mechanism forming ATP directly in an
enzyme-catalyzed reaction - ATPase
- ADP Pi 31 kJ/mole ATP
- This is called Phosphorylation... The opposites
is called Dephosphorylation - A single muscle cell uses 600 million ATP per
minute - The body consumes its own mass in ATP per day via
constant recycling!
19
(No Transcript)
20
Oxidative Phosphorylation
- ATP formed in-directly
- Uses redox rxns (see previous slides)
- NADH
- FADH2
- These molecules harvest energy and transfer it to
ATP by the end of Cellular Resp.
21
STEP 1 - Glycolysis
- A glucose is broken down into 2 Pyruvate
molecules - Brief overview...
- http//highered.mcgraw-hill.com/sites/0072507470/s
tudent_view0/chapter25/animation__how_glycolysis_w
orks.html - Occurs in the cytoplasm
- Anaerobic (doesnt need oxygen!)
- See handout!!
22
(No Transcript)
23
(No Transcript)
24
Glycolysis cont. Recall that there are 2 GAP per
glucose.
25
Glycolysis
- Balance sheet for P bonds of ATP
- How many ATP P bonds expended? ________
- How many P bonds of ATP produced? (Remember
there are two 3C fragments from glucose.)
________ - Net production of P bonds of ATP per glucose
________
2
4
2
26
Overall Equation
- Glucose 2 ADP 2 Pi 2 NAD
- 2 Pyruvate 2 ATP 2 (NADH
H)
27
Is Glycolysis Efficient?
- 2.2 of E from glucose is transferred to ATP via
Glycolysis. - This might be good enough for some
micro-organisms but not larger species like
ourselves! - A much more detailed look...
- http//www.youtube.com/watch?vO5eMW4b29rgfeature
related - Page 115 1-7
28
Mitochondrion Anatomy
29
STEP 2 -Pyruvate Oxidation
- Occurs in the Matrix
- See P 100 for a great diagram
- General Equation...
- CoA coenzyme A
- 2 Pyruvate 2 NAD 2 CoA
- 2 acetylCoA 2 NADH 2H 2CO2
- Acetyl CoA then enters the Kreb Cycle!!
30
STEP 3 - Krebs Cycle
- In mitochondrion
- Mostly on inner membrane
- Many enzymes, coenzymes and other molecules are
in an organize pattern on the inner membrane. - Brief Overview...
- http//highered.mcgraw-hill.com/sites/0072507470/s
tudent_view0/chapter25/animation__how_the_krebs_cy
cle_works__quiz_1_.html
31
Krebs Cycle
- More depth!
- http//www.youtube.com/watch?vA1DjTM1qnPM
- Note where H2O is used and CO2 is released!
32
The Balance Sheet so far...
- By the end of the Krebs Cycle (thru Steps 1-3)
the entire glucose molecule is consumed. - 6C get converted to 6 CO2 along the way!
- HARNESSED ENERGY (NET)!
- 4 ATP (2 Glycolysis, 2 Krebs)
- 12 reduced coenzymes
- 2 NADH (Glycolysis)
- 2 NADH (Pyruvate Oxidation stage)
- 6 NADH (Krebs)
- 2FADH2 (Krebs)
33
(No Transcript)
34
STEP 4 The Electron Transport Chain
35
ETC Details...
- Occurs on the inner membrane of mito
- Transports electrons (from NADH and FADH2) thru a
series of redox rxns that release free energy. - This free energy is used to pump H protons into
the inner membrane space of the mitochondria - This creates an electro-chemical gradient that is
a source of free energy which is used to create
ATP!
36
The Key Structures...
37
ETC
- Overheads
- Visuals...
- http//highered.mcgraw-hill.com/sites/0072507470/s
tudent_view0/chapter25/animation__electron_transpo
rt_system_and_atp_synthesis__quiz_1_.html - http//www.youtube.com/watch?v0LcWbKOW0u8feature
related
38
The Importance of Oxygen
- Oxygen is the final acceptor of electrons that
pass thru the ETC!! - Its high electronegativity pulls the electrons
through the ETC - Electrons fall (like a skydiver)...this energy
pumps H ions into the inner membrane space so
they can fall back into the matrix and make
ATP!
39
Chemiosmosis
- Protons move through a Proton Channel and
- ATP synthase to produce ATP molecules
- Oxidative Phosphorylation!!
- Electrochemical Gradient must be maintained (by
eating!) or ATP production stops!
40
Protons (indicated by charge) enter back into
the mitochondrial matrix through channels in ATP
synthase enzyme complex. This entry is coupled to
ATP synthesis from ADP and phosphate (Pi)
41
What happens to the NADH from Glycolysis?
- NADH diffuses thru the inner membrane via the
glycerol-phosphate shuttle (P105) - NADH passes electrons to FAD to make FADH2
- NADH can also pass electrons to NAD in the matix
via the aspartate shuttle (less common!)
42
NADH vs. FADH2
- In simplified terms NADH pumps 3 H ions
across...therefore creating 3 ATP molecules! - FADH2 enters the ETC at Q...therefore only
pumping 2 H ions across and making 2 ATP
molecules!
43
Aerobic Respiration Efficiency
- Theoretical and Actual Yields
- Actual...depends on environment (ie temp)
- Theoretical 36 ATP
- Actual yield is less...heat loss, H ions
leaking...Approx 30 ATP?? - Aerobic C.R. Is approx 32 efficient
44
Final Balance Sheet
- See page 110 and page 114
- To review all 4 Steps...See these interactive
animations... - http//www.science.smith.edu/departments/Biology/B
io231/ - Page 115 - 8-18
45
Metabolic Rate
- An organisms Metabolic rate is the amount of
energy consumed at a given time and a measure of
the overall rate of C.R. Rxns!
46
Control Mechanisms (p 113)
- Phosphofructokinase (catalyzes step 3 of
Glycolysis) controls C.R. - It is activated by ADP and inhibited by ATP
- NADH inhibits pyruvate decarboxylase and prevents
Acetyl-CoA from forming - An organisms Metabolic Rate is the amount of
energy consumed by an organism in a given time.
47
Related Pathways
48
Protein Catabolism
- PROs Broken down into individual A.A.s in the
body. - First stage of this is deamination (removal of
amino group as ammonia NH3), a waste. - The remaining parts of the A.A.s are converted
into components of glycolysis or Krebs cycle
49
(No Transcript)
50
(No Transcript)
51
Lipid Catabolism
- Triglycerides are digested into glycerol and
fatty acids - Glycerol can be converted into glucose by
gluconeogenesis or into DHAP - Fatty Acids are transported to the matrix,
undergo beta-oxidation (conversion into acetyl
CoA...enters the Kreb cycle)
52
Anaerobic Respiration No O2
53
Lactic Acid Fermentation
- Occurs during vigorous exercise when O2 is in
short supply. Very inefficientbut quick! - After glycolysis, the pyruvic acid is converted
into lactic acid. - L.A. is toxic and must be removed by delivering
O2 to the cellsthis is why you suck wind after a
sprint!!!
54
Lactic Acid Fermentation
- Elite athletes can tolerate higher L.A. levels in
their blood. - Eg. Lance Armstrong 4x the normal threshold!
- This process is also used to make cheese and
yogurtbacteria do the work!
55
Lactate Threshold
- Can be increased by training
56
VO2 Max Test
- A measure of aerobic fitness
- Maximum volume of oxygen (mL) that the cells can
remove from the bloodstream in one minute per kg
of body weight. - 35 mL/kg/min is average
- A very painful test....run/bike faster and faster!
57
VO2 Max
- Can be improved with training
- Genetics!
- Decreases with age
- http//www.youtube.com/watch?vFSL1jkwmWcs
58
Ethanol Fermentation
- Occurs in cytoplasm of yeast cells.
- After glycolysis, pyruvic acid is converted to
CO2 and alcohol. - Ethanol could be a valuable, clean burning fuel
for industry and transportation.
59
Anaerobic Pathways
- Overheads
60
Complimentary Processes
- The energy that fuels life on earth cycles
between P.S. and C.R. - The products of each process become the
substrates for the other. - HW Page 124
- 1-12
61
Lab Page 131
- http//walking.about.com/library/cal/ucrockport.ht
m