Extreme cases: ionic compounds (LiF) - PowerPoint PPT Presentation
Title:
Extreme cases: ionic compounds (LiF)
Description:
Title: Lecture 2 Author: GWH28-DGCMP-P6RC4-6J4MT-3HFDY Last modified by: Authorized User Created Date: 1/31/2006 12:48:03 PM Document presentation format – PowerPoint PPT presentation
Number of Views:54
Avg rating:3.0/5.0
Title: Extreme cases: ionic compounds (LiF)
1
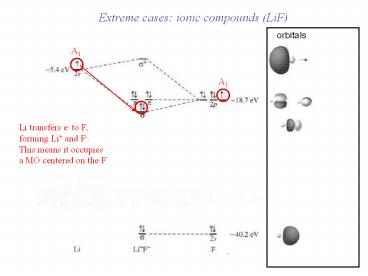
Extreme cases ionic compounds (LiF)
orbitals
2
Molecular orbitals for larger molecules
1. Determine point group of molecule (if linear,
use D2h and C2v instead of D8h or C8v)
2. Assign x, y, z coordinates (z axis is
higher rotation axis if non-linear y axis in
outer atoms point to central atom)
3. Find the characters of the reducible
representation for the combination of 2s orbitals
on the outer atoms, then for px, py, pz. (as for
vibrations, orbitals that change position 0,
orbitals that do not change 1 and orbitals
that remain in the same position but change sign
-1)
4. Find the irreducible representations (they
correspond to the symmetry of group orbitals,
also called Symmetry Adapted Linear
Combinations SALCs of the orbitals).
5. Find AOs in central atom with the same
symmetry
6. Combine AOs from central atom with those
group orbitals of same symmetry and similar E
3
F-H-F- D8h, use D2h
1st consider combinations of 2s and 2p orbitals
on F atoms
Obtain the reducible rep based on equivalent F 2s
orbitals.
G2s 2 2 0 0 0 0 2 2
Use Reduction Procedure to get the irreducible
reps. G2s Ag B1u
Use the Projection Operator to obtain a SALC for
each irreducible rep
Repeat for each group of equivalent atomic
orbitals to obtain the full set of eight SALC.
4
SALC can now be treated similarly to the atomic
orbitals and combined with appropriate AOs from H
1s(H) is Ag so it matches two SALC. The
interaction can be bonding or antibonding.
Both interactions are symmetry allowed, how about
energies?
5
Orbital potential energies (see also Table 5-1 in
p. 134 of textbook)
Average energies for all electrons in the same
level, e.g., 3p (use to estimate which orbitals
may interact)
6
-13.6 eV
-18.65 eV
-40.2 eV
7
Characterize the electrons bonding, non-bonding,
antibonding.
8
CO2 D8h, use D2h
(O O) group orbitals the same as for (F F)!!
But C has more AOs to be considered than H !
9
CO2 D8h, use D2h
Carbon orbitals
10
Ag-Ag interactions of C 2s and the SALC of O 2s
-19.43 eV
-32.38 eV
11
Ag-Ag interactions, now C 2s and the Ag SALC of
the C 2pz
-10.66 eV
-19.43 eV
12
B1u-B1u interactions. Carbon pz with SALC of
oxygen 2s
SALC
13
B1u-B1u interactions. Carbon pz with oxygen pz
SALC
14
All four are symmetry allowed
15
B1u-B1u interactions
Ag-Ag interactions
All four are symmetry allowed
16
Symmetry allows many interactions. Energy
considerations guide as to which is important.
Primary B1u interaction
Primary Ag interaction
SALC of Ag and B1u
SALC of Ag and B1u
Strengths of Interactions
Ag 2s(C) -15.9 --- SALC of 2s(O) 32.4 D
16.5 vs 2s(C) ) -19.4 --- SALC of 2p(O)
-15.9 D 3.5
B1u 2pz(C) -10.7 --- SALC of 2s(O) -32.4 D
21.7 vs 2pz(C) -10.7 --- SALC 2p(O)
-15.9 D 5.2
17
(No Transcript)
18
(No Transcript)
19
LUMO
The frontier orbitals of CO2
HOMO
20
Molecular orbitals for larger molecules H2O
21
2
0
0
2
For H H group orbitals
G A1 B1
E two orbitals unchanged
C2 two orbitals interchanged
sv two orbitals unchanged
sv two orbitals interchanged
22
(No Transcript)
23
a1 sym
b1 sym
b2 sym
24
Molecular orbitals for NH3
Find reducible representation for 3Hs
G
1
0
3
Irreducible representations G A1 E
25
(No Transcript)
26
(No Transcript)
27
Acid-base and donor-acceptor chemistry Hard and
soft acids and bases
28
Classical concepts
- Arrhenius
- acids form hydrogen ions H (hydronium, oxonium
H3O) in aqueous solution - bases form hydroxide ions OH- in aqueous
solution - acid base ? salt water
- e.g. HNO3 KOH ? KNO3 H2O
- Brønsted-Lowry
- acids tend to lose H
- bases tend to gain H
- acid 1 base 1 ? base 1 acid 2 (conjugate
pairs) - H3O NO2- ? H2O HNO2
- NH4 NH2- ? NH3 NH3
- In any solvent, the reaction always favors the
formation of the weaker acids or bases
The Lewis concept is more general and can be
interpreted in terms of MOs
29
Remember that frontier orbitals define the
chemistry of a molecule
CO is a s-donor and a p-acceptor
30
Acids and bases (the Lewis concept)
A base is an electron-pair donor An acid is an
electron-pair acceptor
Lewis acid-base adducts involving metal ions are
called coordination compounds (or complexes)
31
Frontier orbitals and acid-base reactions
Remember the NH3 molecule
32
Frontier orbitals and acid-base reactions
The protonation of NH3
(C3v)
(Td)
33
In most acid-base reactions HOMO-LUMO
combinations lead to new HOMO-LUMO of the product
But remember that there must be useful overlap
(same symmetry) and similar energies to form new
bonding and antibonding orbitals
What reactions take place if energies are very
different?
34
Frontier orbitals and acid-base reactions
Even when symmetries match several reactions are
possible, depending on the relative energies
35
Frontier orbitals and acid-base reactions
Very different energies like A-B or A-E no
adducts form
Similar energies like A-C or A-D adducts form
A base has an electron-pair in a HOMO of suitable
symmetry to interact with the LUMO of the acid
36
The MO basis for hydrogen bonding
F-H-F-
37
MO diagram derived from atomic orbitals (using
F.F group orbitals H orbitals)
38
But it is also possible from HF F-
First form HF
39
The MO basis for hydrogen bonding
F-H-F-
LUMO
HOMO
HOMO
First take bonding and antibonding combinations.
40
Similarly for unsymmetrical B-H-A
Total energy of B-H-A lower than the sum of the
energies of reactants
41
(No Transcript)
42
Hard and soft acids and bases
Hard acids or bases are small and
non-polarizable Soft acids and bases are larger
and more polarizable Halide ions increase in
softness fluoride lt chlorideltbromideltiodi
de Hard-hard or soft-soft interactions are
stronger (with less soluble salts) than
hard-soft interactions (which tend to be more
soluble).
43
Most metals are classified as Hard (Class a)
acids or acceptors. Exceptions shown below
acceptors metals in red box are always soft
(Class b). Other metals are soft in low
oxidation states and are indicated by symbol.
Class (b) or soft always
Solubilities AgF gt AgCl gt AgBr gtAgI But
LiBr gt LiCl gt LiI gt LiF
44
Chatts explanationClass (b) soft metals have d
electrons available for p-bonding
Model Base donates electron density to metal
acceptor. Back donation, from acid to base, may
occur from the d electrons of the acid metal into
vacant orbitals on the base.
Higher oxidation states of elements to the right
of transition metals have more class b
character since there are electrons outside the d
shell. Ex. (Tl(III) gt Tl(I), has two 6s
electrons outside the 5d making them less
available for p-bonding)
For transition metals high oxidation states and
position to the left of periodic table are
hard low oxidation states and position to the
right of periodic table are soft
Soft donor molecules or ions that are readily
polarizable and have vacant d or p
orbitals available for p-bonding react best with
class (b) soft metals
45
(No Transcript)
46
Tendency to complex with hard metal ions N gtgt P
gt As gt Sb O gtgt S gt Se gt Te F gt Cl gt Br gt I
Tendency to complex with soft metal ions N ltlt P
gt As gt Sb O ltlt S gt Se Te F lt Cl lt Br lt I
47
The hard-soft distinction is linked to
polarizability, the degree to which a molecule or
ion may be easily distorted by interaction with
other molecules or ions.
Hard acids or bases are small and
non-polarizable Soft acids and bases are larger
and more polarizable
Hard acids are cations with high positive charge
(3 or greater), or cations with d electrons not
available for p-bonding
Soft acids are cations with a moderate positive
charge (2 or lower), Or cations with d electrons
readily availbale for p-bonding
The larger and more massive an ion, the softer
(large number of internal electrons Shield the
outer ones making the atom or ion more
polarizable)
For bases, a large number of electrons or a
larger size are related to soft character
48
Hard acids tend to react better with hard bases
and soft acids with soft bases, in order to
produce hard-hard or soft-soft combinations In
general, hard-hard combinations are
energetically more favorable than soft-soft
An acid or a base may be hard or soft and at the
same time it may be strong or weak Both
characteristics must always be taken into
account e.g. If two bases equally soft compete
for the same acid, the one with greater basicity
will be preferred but if they are not equally
soft, the preference may be inverted
49
Fajans rules
- For a given cation, covalent character increases
- with increasing anion size. FltClltBrltI
- For a given anion, covalent character increases
- with decreasing cation size. KltNaltLi
- The covalent character increases
- with increasing charge on either ion.
- Covalent character is greater for cations with
non-noble gas - electronic configurations.
A greater covalent character resulting from a
soft-soft interaction is related to lower
solubility, color and short interionic
distances, whereas hard-hard interactions result
in colorless and highly soluble compounds
50
(No Transcript)
51
Quantitative measurements
Absolute hardness (Pearson)
Mullikens absolute electronegativity (Pearson)
EHOMO -I ELUMO -A
Softness
52
- Energy levels
- for halogens
- and relations between
- , h and HOMO-LUMO energies
53
(No Transcript)