Pr PowerPoint PPT Presentation
Title: Pr
1
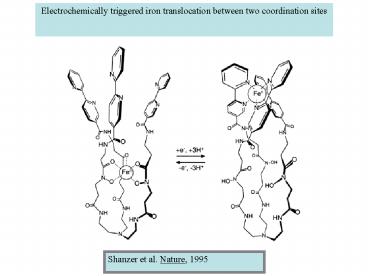
Electrochemically triggered iron translocation
between two coordination sites
Shanzer et al. Nature, 1995
2
Redox-Driven Anion Translocation between Metal
Centers
Controlled motion can be generated at the
molecular level by making a particle translocate
between fixed positions, following a prescribed
pathway. The particle can be an anion, X-, and
can be made to move between two transition metal
centers, M1 and M2, taking advantage of the redox
activity of one of them.
3
(No Transcript)
4
square scheme illustrating the redox-driven
translocation of an X- anion from Cu(II) to
Ni(III) and vice versa. As the transient species
b has too short a lifetime, the oxidation step,
OX, and the direct translocation step, Tdir,
cannot be distinguished and are perceived as
simultaneous. The same happens for the reduction
(RED) and reverse translocation (Trev) steps.
5
Electrochemically triggered anion (Cl-)
translocation in a multicentric supramolecular
coordination compound.
6
Transition metal ions can be moved reversibly
between the two coordinatively unequivalent
compartments A and B of a ditopic ligand, using
as an input the variation of a bulk solution
parameter, either pH or redox potential. In a
redox-driven translocation, the metal moves
reversibly from A to B on cycling between
two consecutive oxidation states (e.g.,
Cu(II)/Cu(I) Fe(III)/Fe(II)) by means of
auxiliary oxidation and reduction reactions. In a
pH-driven process, one compartment displays also
acid-base properties.
7
Redox-driven translocation of a metal ion. The
oxidized cation,M( n1) (smaller sphere), has a
hard nature and likes staying in the hard
compartment A. The reduced cation,Mn (larger
sphere), of soft characteristics, prefers to
reside in the soft compartment B. The metal
center can be reversibly translocated between A
and B, through the M( n1)/Mn redox cycle,
coupled to an auxiliary oxidation-reduction
process.
8
(No Transcript)
9
Redox-driven translocation of a copper center,
based on the Cu(II)/Cu(I) change. The Cu(II) ion
stays in the tetraamine compartment of the
ditopic ligand, whereas the Cu(I) ion prefers to
occupy the bis(2,2-bipyridine) compartment. The
fast and reversible translocation of the metal
between the two compartments can be carried out
through auxiliary redox processes (reduction of
Cu(I)I with ascorbic acid oxidation of Cu(I)
with H2O2).
10
Cd(II) and Pb(II) Complexation by
Dipyridine-Containing Macrocycles with Different
Molecular Architecture. Effect of Complex
Protonation on Metal Coordination Environment
Paloma Arranz, Carla Bazzicalupi, Andrea
Bencini,, Antonio Bianchi,, Samuele
Ciattini, Patrizia Fornasari, Claudia Giorgi,
and Barbara Valtancoli
Inorg. Chem. 2001, 40, 6383-6389
11
redox-driven translocation of the copper
cation Shanzer, Albrecht-Gary and coworkers
(Chem. Comm. 2002)
12
kI 2 x 10-2 s-1
kII 9 x 10-4 s-1