Questions of the Day: - PowerPoint PPT Presentation
1 / 12
Title:
Questions of the Day:
Description:
... Cracking of dimer Diels Alder: Reaction of 1,3-cyclopentadiene with maleic anhydride Hydrolysis of Diels-Alder product Dimer D.- A ... – PowerPoint PPT presentation
Number of Views:129
Avg rating:3.0/5.0
Title: Questions of the Day:
1
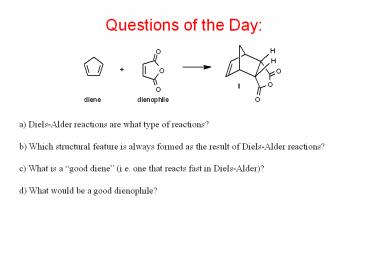
Questions of the Day
a) Diels-Alder reactions are what type of
reactions? b) Which structural feature is always
formed as the result of Diels-Alder reactions?
c) What is a good diene (i.e. one that reacts
fast in Diels-Alder)? d) What would be a good
dienophile?
2
- Today
- Diels-Alder (cont.), NMR
- Esterification (Exp.5), a first introduction
- MiniQuiz
3
Diels-Alder reactions require a 1,3-diene (in
s-cis) and a dienophile (an alkene, best when
EWG is attached).
Reaction equations for our experiment Retro
Diels Alder Cracking of dimer Diels Alder
Reaction of 1,3-cyclopentadiene with maleic
anhydride Hydrolysis of Diels-Alder product
Dimer
D.-A. adduct Cis endo
- Compare
- Polarity of I versus II
- Mp. of I versus II
4
Diels Alder Reactions
Which of the following dienes would be most
reactive? why? 1,3-cyclopentadiene, 1,3-butadiene
(see problems on Report Form) Which of the
following dienophiles would be more reactive?
Ethene or maleic anhydride
5
Diels Alder Reactions
Synthesis of Natural Products
D
C
Steroids!
A
B
6
CH3
CH3
D
C
CH3
CH3
A
B
HO
Cholesterol
7
Diels Alder
The H-NMR of the dimer of 1,3-cyclopentadiene is
quite complex, with many signals. In contrast,
the proton NMR of our Diels-Alder product is
simple, with few signals the same is true for
its hydrolysis product. How can you explain this?
8
Methyl Benzoate Synthesis (Exp.5) the first step
of a two- step synthesis
You will heat your reaction under reflux for 1
hour! Do Practice Problems, calculations
- In order to increase the yield of the ester,
which of the following would help? - use an excess of methanol
- use an excess of conc. sulfuric acid
- Calculations !
Why sulfuric acid and not HCl?
9
Esters and Esterifications
- General properties of esters?
- Compare the boiling points of carboxylic acids
and esters of similar molecular weight and
explain. - Examples of esters in biological systems?
- Fats and Oils
- Waxes
- Ripening process in fruits
- phosphate esters in DNA
10
Salicin and Aspirin
Salicin in willow bark (Salix)
11
- Next time
- Conclusion of Esterification (Exp. 5)
- Grignard reactions (Exp. 6)
- MiniQuiz on todays class
12
MiniQuiz 4 Problems
- Draw a simple, but clear H-NMR for alpha -
monobromoethylbenzene - Draw a simple, but clear H-NMR for alpha -
dibromoethylbenzene