Ionic Compounds PowerPoint PPT Presentation
1 / 20
Title: Ionic Compounds
1
Ionic Compounds
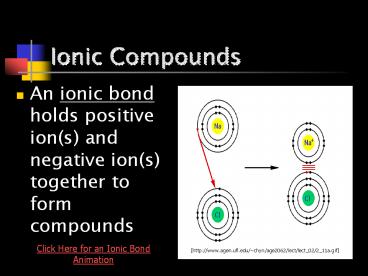
- An ionic bond holds positive ion(s) and negative
ion(s) together to form compounds
Click Here for an Ionic Bond Animation
http//www.agen.ufl.edu/chyn/age2062/lect/lect_0
2/2_11a.gif
2
Ionic Compounds
- Most are compounds that contain a metal and a
non-metal. - Ex NaCl
3
Ionic Compounds
- Most form crystal structures.
- Can be very brittle and will break or shatter on
contact.
http//liftoff.msfc.nasa.gov/academy/materials/to
paz.gif
4
Ionic Compounds
- Most have high melting points. (Very Strong
Bonds!) - Most will dissolve easily in water
- Will conduct electricity when liquid or dissolved
in H2O
5
Ionic Compound Formulas
- Chemical Formulas show how many atoms of a
particular element are present in a compound - Example Al2O3
- 2 Aluminum atoms 3 Oxygen atoms
6
Ionic Compound Formulas
- Ionic formulas are determined by the positive and
negative charges of the atoms.
7
http//wps.prenhall.com/wps/media/objects/602/616
516/Media_Assets/Chapter02/Text_Images/FG02_11.JPG
8
Ionic Compound Formulas
- Example Magnesium Chloride
- Mg2 and Cl-1
- You need 2 Cl-1 to neutralize the Mg2
- MgCl2
http//www.southwest.com.au/jfuller/chemistry/ma
gnesiumchloride.gif
9
Ionic Compound Formulas
- Use the Criss-Cross method to write formulas
- Ex Sodium Sulfide
Na1
S-2
Na
S
2
10
Ionic Compound Formulas
- Ex Magnesium Oxide
- Mg2 and O-2
- Criss-Cross gets Mg2O2
- But only 1 atom of each is needed to neutralize
so MgO is correct!
11
Ionic Compound Names
- 1st - Write the name of the metallic element.
- 2nd Then write the name of the non-metallic
element with the suffix ide.
12
Ionic Compound Names
- Example MgBr2
- Magnesium Bromide
- Example Al2O3
- Aluminum Oxide
13
Transition Metal Ions
- Transition metals do not have an ion pattern and
can have more than one type of charge.
http//wps.prenhall.com/wps/media/objects/602/616
516/Media_Assets/Chapter02/Text_Images/FG02_12.JPG
14
(No Transcript)
15
Transition Metal Ions
- Example FeO
- What is the charge of Fe?
- We know O-2
- Fe must be 2 to neutralize the 2 of Oxygen...
- Iron (II) Oxide
16
Transition Metal Ions
- Example Cu2O
- Copper (I) Oxide
17
Polyatomic Ions
- Ions that are made of multiple atoms.
- Example OH- is the hydroxide ion.
- Pg. 232
18
(No Transcript)
19
Polyatomic Ions
- When naming, use the exact name of the ion.
- Example NaNO3
- This is sodium nitrate.
20
Polyatomic Ions
- Example Magnesium Hydroxide
- Mg2 and OH-
- Mg OH 2
( )