Carboxylic Acids, Esters, Amines, and Amides - PowerPoint PPT Presentation
1 / 62
Title:
Carboxylic Acids, Esters, Amines, and Amides
Description:
* Focus on the Human Body Epinephrine and Related Compounds A hormone is a ... Amines Structure and Classification Amines are organic nitrogen compounds, ... – PowerPoint PPT presentation
Number of Views:292
Avg rating:3.0/5.0
Title: Carboxylic Acids, Esters, Amines, and Amides
1
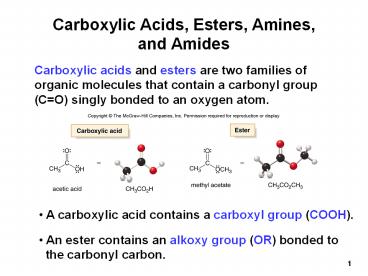
Carboxylic Acids, Esters, Amines, and Amides
Carboxylic acids and esters are two families of
organic molecules that contain a carbonyl group
(CO) singly bonded to an oxygen atom.
- A carboxylic acid contains a carboxyl group
(COOH).
- An ester contains an alkoxy group (OR) bonded to
- the carbonyl carbon.
1
2
Carboxylic Acids, Esters, Amines, and Amides
Amines and amides are nitrogen-containing organic
molecules.
- An amine is an organic nitrogen compound
- formed by replacing one or more hydrogen atoms
- of ammonia (NH3) with alkyl groups.
- An amide is a carbonyl compound that contains a
- nitrogen atom bonded to the carbonyl carbon.
2
3
NomenclatureNaming a Carboxylic Acid (RCOOH)
To name a carboxylic acid using the IUPAC system
- Find the longest chain containing the COOH
- group, and change the -e ending of the parent
- alkane to -oic acid.
- Number the chain to put the COOH group at C1,
but - omit 1 from the name. Apply all other
- nomenclature rules.
CH3
hexane ? hexanoic acid
CH3CHCHCH2CH2
OH
Answer 4,5-dimethylhexanoic acid
CH3
1
4
5
4
NomenclatureNaming a Carboxylic Acid (RCOOH)
- Many simple carboxylic acids are referred to by
- their common names. A common name uses the
- suffix -ic acid.
formic acid
acetic acid
benzoic acid
4
5
NomenclatureNaming an Ester (RCOOR)
To name an ester using the IUPAC system
- Name the R' group bonded to the oxygen atom as
- an alkyl group.
- Name the RCO group by changing the -ic acid
- ending of the parent carboxylic acid to the
- suffix -ate.
5
6
NomenclatureNaming an Ester (RCOOR')
Sample Problem 13.2
Give the IUPAC name for the following ester
Name the R group bonded to the O atom as an
alkyl group.
Step 1
methyl group
6
7
NomenclatureNaming an Ester (RCOOR')
Sample Problem 13.2
Step 2
Name the acyl group (RCO-) by
- Changing the -ic acid ending of the
parent carboxylic acid to -ate.
- This becomes the second part of the name.
Answer methyl butanoate
derived from butanoic acid ? butanoate
7
8
Physical Properties of Carboxylic Acids and Esters
- Carboxylic acids and esters are polar compounds.
- Only carboxylic acids can undergo intermolecular
- hydrogen bonding.
8
9
Physical Properties
Carboxylic acids also have higher boiling
points than esters because esters are incapable
of intermolecular hydrogen bonding.
methyl acetate
propanoic acid
Increasing boiling point
9
10
Physical Properties
Carboxylic acids have higher boiling points than
similar alcohols because there are more
hydrogen bonding interactions possible than
alcohols.
Increasing boiling point
10
11
Interesting Carboxylic Acids
- Simple carboxylic acids have foul or biting
odors.
- Formic acid (HCO2H) is responsible for the sting
- of some types of ants.
- Acetic acid (CH3CO2H) is the sour-tasting
- component of vinegar it can be made by air
- oxidation of ethanol when wine goes bad.
11
12
Focus On Health MedicineSkin Care Products
- Several skin care products purported to smooth
- fine lines contain a-hydroxy acids.
- General structure of an a-hydroxy acid
- These acids work by removing the outer, older
layer - of skin cells, revealing the healthier looking,
new - cells underneath.
12
13
Focus On Health Medicine Aspirin and
Anti-Inflammatory Agents
- Common pain relievers that are also anti-
- inflammatory agents contain a carboxyl group
aspirin
ibuprofen
13
14
Interesting Carboxylic AcidsAspirin and
Anti-Inflammatory Agents
- Pain relievers work because they block the
- synthesis of prostaglandins, compounds
- responsible for pain responses in the body.
14
15
The Acidity of Carboxylic Acids
- Carboxylic acids are proton (H) donors
- They are weak acids compared to inorganic acids
- like HCl or H2SO4.
- Only a small percentage of a carboxylic acid is
- ionized in aqueous solution.
15
16
The Acidity of Carboxylic AcidsReaction with
Bases
- Carboxylic acids react with bases such as NaOH
- to form water-soluble salts.
- A proton is removed from acetic acid to form its
- conjugate base, the acetate anion, which is
present - in solution as its sodium salt, sodium acetate.
- Hydroxide (OH-) gains a proton to form neutral
H2O.
16
17
The Acidity of Carboxylic AcidsCarboxylate
AnionsSalts of Carboxylic Acids
- The salts of carboxylic acids formed by
acid-base - reactions are water-soluble ionic solids.
- Thus, the reaction will turn a water-insoluble
- carboxylic acid into a water-soluble carboxylic
- acid salt.
O
O
CH3(CH2)6COH octanoic acid
Na -OH base
CH3(CH2)6CO- Na sodium octanoate
HOH
Water-insoluble
Water-soluble
17
18
The Acidity of Carboxylic AcidsHow Does Soap
Clean Away Dirt?
- Soap has been used by humankind for 2000 years.
- Soaps are salts of carboxylic acids that have
- many C atoms in a long hydrocarbon chain.
- A soap molecule has two parts
- The ionic end is called the polar head.
- The carbon chain of nonpolar CC and CH bonds is
called the nonpolar tail.
18
19
The Acidity of Carboxylic AcidsHow Does Soap
Clean Away Dirt?
- Structure of a soap molecule
polar head ionic end
nonpolar tail nonpolar end
19
20
The Acidity of Carboxylic AcidsHow Does Soap
Clean Away Dirt?
- In water, soap forms micelles, spherical
droplets - with the ionic heads on the surface and the
nonpolar - tails packed together in the interior.
20
21
The Acidity of Carboxylic AcidsHow Does Soap
Clean Away Dirt?
- Nonpolar hydrocarbon tails trap nonpolar
material - like grease and oil.
- The polar head remains on the surface, sealing
- off the grease and oil, and washing away in
- the presence of water.
21
22
Reactions Involving Carboxylic Acidsand Esters
- Carboxylic acids and esters undergo a common
- type of reactionsubstitution.
22
23
Reactions Involving Carboxylic Acidsand
EstersEster Formation
- Carboxylic acids react with alcohols to form
- esters.
- Here, the OR' group replaces the OH group.
23
24
Reactions Involving Carboxylic Acidsand
EstersEster Formation
- Formation of an ester is done by the Fisher
- esterification
24
25
Reactions Involving Carboxylic Acidsand
EstersEster Hydrolysis
- Treatment of an ester (RCOOR') with water in the
- presence of an acid catalyst forms a carboxylic
- acid (RCOOH) and a molecule of alcohol (R'OH).
- Here, the OH group replaces the OR' group.
25
26
Reactions Involving Carboxylic Acidsand
EstersEster Hydrolysis
- This reaction is a hydrolysis, since bonds are
- cleaved by reaction with water.
26
27
Focus on Health MedicineOlestra, a Synthetic
Fat
- Triacylglycerols, common naturally occurring
- esters, contain three ester groups, each with a
long - C chain.
- They are lipids, water-insoluble organic
- compounds, present in fats and oils.
27
28
Focus on Health MedicineOlestra, a Synthetic
Fat
- Animals store energy in the form of
triacylglycerols.
- Using enzymes called lipases, the three ester
- bonds are hydrolyzed when the triacylglycerol
is - metabolized.
- Diets high in fat lead to obesity, so the fake
fat - olestra (Olean) was invented to replace some
- triacylglycerols in snack foods.
- The lipase enzymes cannot hydrolyze the olestra,
- so it passes through the body unmetabolized,
- providing no calories to the consumer.
28
29
Focus on Health MedicineOlestra, a Synthetic
Fat
- Hydrolysis of a triacylglycerol by lipase
O
HO
C
R
CH2O
R
CH2OH
H2O
R
R
CH O
HO
CH OH
lipase
HO
R
CH2O
R
CH2OH
glycerol
3 long-chain carboxylic acids
The 3 bonds that break are drawn in red.
29
30
Focus on Health MedicineStructure of Olestra
30
31
AminesStructure and Classification
- Amines are organic nitrogen compounds, formed by
- replacing one or more hydrogen atoms of ammonia
- (NH3) with alkyl groups.
- Amines are classified by the number of alkyl
groups - bonded to the N atom.
- A primary (1o) amine contains
- 1 CN bond, and has the
- general structure RNH2.
31
32
AminesStructure and Classification
- A secondary (2o) amine
- contains 2 CN bonds, and
- has the general structure R2NH.
- A tertiary (3o) amine has
- 3 CN bonds, and has the
- general structure R3N.
- The 1o, 2o, and 3o amine N atom has a lone pair
of - e-, which is omitted in condensed structures.
32
33
AminesStructure and Classification
- The amine N has a trigonal pyramidal shape,
- with bond angles of 109.5o.
33
34
AminesStructure and Classification
- The amine N atom can also be part of a ring.
- Morphine and atropine each contains a nitrogen
- atom in a ring.
- They are alkaloidsnaturally occurring amines
- derived from plant sources.
34
35
NomenclatureAmines
- To name a primary (1o) amine, name the alkyl
group - bonded to the nitrogen atom and add the
- suffix -amine.
- For 2o and 3o amines with different alkyl
groups, - alphabetize the names of the alkyl groups.
- 2o and 3o amines with identical alkyl groups are
- named using the prefix di- or tri-
35
36
NomenclatureAmines
Sample Problem 13.8
Name each amine.
a.
For a 1amine, name the alkyl group and add the
suffix -amine.
Answer pentylamine
36
37
NomenclatureSecondary and Tertiary Amines
Sample Problem 13.8
b.
For a 2amine, name each alkyl group, alphabetize
the names, and add the suffix -amine.
Answer ethylmethylamine
37
38
NomenclatureAromatic Amines
- Amines with the N directly bonded to a benzene
- ring are named as derivatives of aniline.
- Use the prefix N- before any alkyl group bonded
- to the amine nitrogen.
38
39
Physical Properties
- Many low molecular weight amines have very foul
- odors like rotting fish, urine, and bad breath.
- Amines are polar molecules, containing either
- polar CN or polar HN bonds.
- 1o and 2o amines can have intermolecular
- hydrogen bonding
39
40
Physical Properties
- 1o and 2o amines have higher boiling points than
- compounds that do not have intermolecular
- hydrogen bonding.
CH3CH2OCH2CH3 diethyl ether bp 38 oC
CH3CH2CH2CH2NH2 butylamine bp 78 oC
Increasing boiling point
40
41
Physical Properties
- 1o and 2o amines have lower boiling points than
- alcohols, as alcohols have stronger
intermolecular - hydrogen bonds.
CH3CH2CH2CH2NH2 butylamine bp 78 oC
CH3CH2CH2CH2OH 1-butanol bp 117 oC
Increasing boiling point
41
42
Physical Properties
- 3o amines have lower boiling points than 1o or
- 2o amines of comparable size because they have
no - NH bonds.
Increasing boiling point
42
43
Amines as Bases
- Amines are bases they are proton (H) acceptors.
- This acidbase reaction occurs with 1º, 2º, and
- 3º amines.
- Amines are weak bases compared to inorganic
- bases like NaOH.
43
44
Amines as BasesReactions of Amines with Acids
- Amines react with acids (HCl) to form water-
- soluble salts.
conjugate acid
conjugate base
base
acid
44
45
Amines as BasesAmmonium Salts
- When an amine reacts with an acid, the product
is an - ammonium salt.
- The amine forms a positively charged ammonium
- ion and the acid forms an anion.
45
46
Amines as BasesAmmonium Salts
To name an ammonium salt
- Change the suffix -amine of the parent
- amine to the suffix -ammonium.
- Add the name of the anion.
Answer triethylammonium acetate
46
47
Amines as BasesAmmonium Salts
- A water-insoluble amine is converted to a water-
- soluble ammonium salt by treatment with acid.
water-insoluble
water-soluble
47
48
Focus on Health MedicineAmmonium Salts as
Useful Salts
- Many amines with medicinal properties are sold
- as their ammonium salts, which are transported
- through the aqueous bloodstream.
- The antihistimine Benadryl is sold as the salt
- diphenhydramine hydrochloride.
48
49
Amides
Amides contain a carbonyl group bonded to a N
atom.
Amides are classified based on the number of
C atoms bonded to the N atom.
- A primary (1o) amide contains
- 1 CN bond.
- It is abbreviated as RCONH2.
49
50
Amides
- A secondary (2o) amide
- contains two CN bonds,
- and is abbreviated RCONHR'.
- A tertiary (3o) amide
- contains three CN bonds,
- and is abbreviated RCONHR'2.
50
51
NomenclatureNaming an Amide
- All 1o amides are named by replacing the -oic
acid - ending of the parent carboxylic acid with
-amide.
NH2
derived from benzoic acid ? benzamide
derived from acetic acid ? acetamide
51
52
NomenclatureNaming an Amide
To name a 2º or 3º amide
- Name the alkyl group (or groups) bonded to
- the N atom of the amide. Use the prefix N-
- preceding the name of each alkyl group.
- Name the RCO group with the suffix -amide.
52
53
NomenclatureNaming an Amide
Sample Problem 13.12
Name the following amide HCONHCH2CH3
Name the alkyl group on the N atom, and precede
its name with N-.
Step 1
Name N-ethyl
ethyl group
53
54
NomenclatureNaming an Amide
Sample Problem 13.12
Step 2
Name the acyl group with -amide.
derived from formic acid ? formamide
Answer N-ethylformamide
54
55
Physical Properties
1o and 2o amides have higher boiling points
than 3o amides and esters because of
intermolecular hydrogen bonding.
Increasing boiling point
55
56
Hydrolysis of Esters and AmidesAmide Hydrolysis
- Treatment of an amide with water in the presence
- of an acid catalyst (HCl) forms a carboxylic
acid - and an ammonium salt.
56
57
Hydrolysis of Esters and AmidesAmide Hydrolysis
- Amides are also hydrolyzed in aqueous base to
- form carboxylate anions and a molecule of
ammonia - (NH3) or amine.
57
58
Interesting Amines and AmidesCaffeine and
Nicotine
- Caffeine and nicotine are widely used stimulants
- of the central nervous system that contain
- nitrogen atoms in rings.
- They are alkaloidsnaturally occurring amines
- derived from plants.
58
59
Focus on the Human BodyEpinephrine and Related
Compounds
- A hormone is a compound produced by an
- endocrine gland, which travels through the
blood- - stream to a target tissue or organ.
- Epinephrine (adrenaline) is a hormone made from
- norepinephrine, the neurotransmitter.
59
60
Focus on the Human BodyEpinephrine and Related
Compounds
- Danger or emotional stress causes the formation
- of epinephrine.
- The body will metabolize stored carbohydrates
- to form glucose, which is further metabolized
to - provide an energy boost.
- The heart rate increases and lung passages are
- dilated.
- This effect is known as a rush of adrenaline
or - fight-or-flight response.
60
61
Focus on Health MedicinePenicillin
- The antibiotic properties of penicillin were
first - discovered in 1928 by Sir Alexander Fleming.
- Penicillin interferes with the enzyme that
- synthesizes bacterial cell walls, killing the
- bacterium.
- All penicillins contain a ß-lactam
61
62
Focus on Health MedicineCommon Penicillin Used
Today
62