Chapter 24 - PowerPoint PPT Presentation
1 / 42
Title:
Chapter 24
Description:
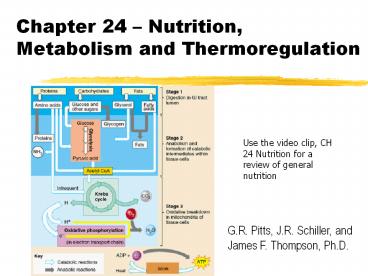
Chapter 24 Nutrition, Metabolism and Thermoregulation Use the video clip, CH 24 Nutrition for a review of general nutrition G.R. Pitts, J.R. Schiller, and – PowerPoint PPT presentation
Number of Views:452
Avg rating:3.0/5.0
Title: Chapter 24
1
Chapter 24 Nutrition, Metabolism and
Thermoregulation
Use the video clip, CH 24 Nutrition for a review
of general nutrition
G.R. Pitts, J.R. Schiller, and James F.
Thompson, Ph.D.
2
Nutrition
- We eat, we digest, we absorb, then what?
- 3 fates for food nutrients
- Most are used to supply energy for life
- Some are used to synthesize structural or
functional molecules - The rest are stored for future use love handles!
3
Nutrition for College Students
Four Groups Grease Salt Sugar Alcohol
4
Weight Management
- If energy consumption (food intake) equals energy
utilized (activity), then body weight will remain
constant - Activity and consumption levels vary day to day,
but individuals keep relatively constant weight
for long periods of time - Many individuals in affluent nations have an
imbalance between intake and use ? obesity
5
Regulation of Food Intake
- Hypothalamus - complex integrating center
receiving sensory information from all parts of
the body - Two hypothalamic centers control eating
- Feeding (hunger) center
- located in lateral hypothalamus
- when stimulated, it initiates feeding, even if
one is full - Satiety center
- ventromedial nuclei
- when stimulated, they cause cessation of eating,
even if one has been starved for days - Controls for their set points are unknown!
6
Regulation of Food Intake (cont.)
- Feeding center is always active but the satiety
center can inhibit it - May be driven by changes in blood composition
- glucostatic theory blood glucose levels vary
- lipostatic theory - blood lipid levels vary fat
released from adipose tissue between meals - Other influences
- blood amino acid levels
- temperature - high temp decreases appetite
- GI tract distension (a slow and variable reflex)
- social and psychological factors
- hormones (CCK), neurotransmitters, ions (Zn)
7
Metabolism
- All biochemical reactions in the body
- Balance between synthesis (anabolic) and
breakdown (catabolic) reactions - Anabolism
- chemical reactions that combine simple, smaller
molecules into more complex molecules - uses energy
- protein formation from amino acids
- carbohydrate formation from simple sugars
- etc.
- Catabolism
- chemical reactions that break down complex
organic molecules into simpler ones - releases energy
- proteins are broken down by various proteases
- etc.
8
Relationship of Catabolism to Anabolism
9
Adenosine Tri-Phosphate
- ATP is the link between anabolism and catabolism
- ATP energy is the currency used in most
cellular energy exchanges - catabolic reactions provide the ATP energy that
most anabolic reactions require - only about 10-30 of the energy released by
catabolic reactions can be used - most chemical energy is lost as waste heat
- waste heat is not wasted it is essential in
maintaining a constant body temperature
3 Phosphates
10
ATP Metabolism
- Allows for transfer of small but useful amounts
of energy from one molecule to another - Cell's entire amount of ATP is recycled
approximately every minute - ATP is NOT for long term energy storage
- too reactive in the cell
- other molecules available for energy storage
(neutral fats, glycogen, creatine phosphate,
etc.) - About 8kg (17 lb) of ATP is produced every hour
in an average male - Total amount of ATP present in the body at any
time is only about 50g
11
ATP Metabolism (cont.)
- energy is released by breaking the third
phosphate groups bond - ATP ? ADP Pi
- a reversible reaction
- the energy released is enough to drive anabolic
reactions - ATP ? ADP CrP
- creatine provides energy storage in skeletal
muscle - allows for more ATP to be formed when O2 is less
readily available during skeletal muscle
contraction
12
Energy Production
- Energy is stored in chemical bonds
- Oxidation-Reduction (Redox) reactions
- Oxidation component
- also known as dehydrogenation reactions
- remove electrons from molecules
- decreases the energy remaining in the oxidized
molecule - generally, 2e- (and 2H) are removed
simultaneously - Can also be the gain of oxygen
13
Energy Production (cont.)
- Oxidation-Reduction reactions (cont.)
- Reduction component
- addition of electrons to a molecule
- increases the energy of the reduced molecule
- These 2 component reactions are always coupled
oxidation-reduction reactions
14
Energy Production (cont.)
- ATP Generation
- Addition of phosphate to a chemical compound is
phosphorylation - 3 mechanisms for this
- (1) substrate-level phosphorylation a
high-energy phosphate group is transferred
directly from a molecule to ADP to make ATP - For example, when the energy stored on a
high-energy phosphate group on creatine phosphate
is transferred to ADP to make ATP in skeletal
muscles
CK transfers its high energy phosphate to ADP
15
Energy Production (cont.)
- ATP Generation
- Adding a phosphate ion to a molecule is
phosphorylation - 3 mechanisms
- (2) oxidative phosphorylation
- electrons (H) removed from molecules
- enzymes combine H with O2 releasing enough
energy for ATP formation - (3) photo-phosphorylation - photosynthesis
16
Carbohydrate Metabolism
- General
- 80 of carbohydrates ingested contain glucose
remainder fructose, galactose - glucose is the body's preferred carbohydrate
energy source - Fate of carbohydrates -- depends on needs of body
cells - ATP production
- Amino acid synthesis
- Glycogenesis
- Lipogenesis
- Excretion in urine (minimal)
17
Carbohydrate Metabolism (cont.)
- Glucose movement into cells
- facilitated diffusion - insulin controls (?) rate
- glucose is immediately phosphorylated upon entry
(glucose-6-phosphate) - maintains the diffusion (concentration) gradient
- traps glucose inside the cell
18
Carbohydrate Metabolism (cont.)
- Glucose anabolism
- Glucose storage glycogenesis
- glycogen formation is stimulated by insulin
- glucose not needed immediately is stored in the
liver (25) and in skeletal muscle (75) - Glucose release glycogenolysis
- converts glycogen to glucose
- occurs between meals, stimulated by glucagon and
epinephrine
19
Carbohydrate Metabolism (cont.)
- Glucose anabolism (cont.)
- Formation of glucose from proteins, fats
gluconeogenesis - when blood glucose level is low, you eat if
glucose remains low, body catabolizes some
proteins and fats - stimulated by cortisol and thyroid hormone
- cortisol (glucocorticoids) mobilizes proteins,
making AA's available - thyroid hormone mobilizes proteins (AA's) and may
mobilize lipids - epinephrine, glucagon, hGH also stimulate
- These five hormones are often referred to as the
insulin antagonists.
20
Glucose Metabolism
- Glucose Catabolism
- glucose oxidation is known as cellular
respiration - complete catabolism of each molecule of glucose
to CO2, H2O - maximum yield of 36 ATP molecules/glucose
- 38 of the energy present in a glucose
- excellent efficiency for a biological system
- the rest of the energy is waste heat
- 2 linked enzymatic pathways are involved in
glucose catabolism - glycolysis
- Krebs cycle
21
Glucose Metabolism (cont.)
- Glycolysis - Overview
- Occurs in cytosol
- 1 glucose ?2 pyruvates (pyruvic acid)
- net gain 2 ATPs
- 2 ATPs used
- 4 ATPs made
- net gain 2 NADH 2H (aerobic conditions)
22
Glucose Metabolism (cont.)
- Fate of pyruvate (pyruvic acid) - depends on
availability of O2 - without O2 NADH H pyruvate ? lactic acid
- with O2 available to the cell
- pyruvate converted to acetyl coenzyme A (acetyl
CoA) - this reaction couples glycolysis to the Krebs
cycle
23
Glucose Metabolism (cont.)
- Pyruvic acid - formation of acetyl coenzyme A
(Acetyl CoA) CO2 - lose one carbon from pyruvate to form CO2 (waste)
- the remaining two carbons, the acetyl group, join
with CoA, to generate NADH H (1 from each
pyruvate 2 NADH 2H total from one
glucose)
24
Glucose Metabolism (cont.)
- Krebs cycle (Citric Acid Cycle or Tricarboxylic
Acid Cycle (TCA) - oxidation of acetyl Coenzyme A
- reduction of coenzymes (NAD, FAD)
- Oxidative phosphorylation
- uses NADH2s and FADH2s to make additional ATPs
25
Glucose Metabolism (cont.)
Glycolysis and Krebs Cycle combined total 6 CO2
(waste) 6 H2O 10 NADH2 2 FADH2 4 ATP
(energy harvest)
26
Electron Transport
- Electrons Source NADH2/FADH2 from glycolysis and
Krebs cycle - High-energy electrons enter the system, and
low-energy electrons leave
Animal Physiology, Hill et al., 2004
27
- Electron Transport System
- Oxidative phosphorylation
- O2 is the final electron acceptor for low-energy
electrons from last of the carrier molecules - NADH2 ? 3 ATP
- FADH2 ? 2 ATP
- Enzyme cytochrome oxidase splits apart O2
molecules - Combines each O atom with 2 H ions to makes H2O
water
Animal Physiology, Hill et al., 2004
28
Glucose MetabolismOverview
- C6H12O6 6 O2 ?
- 6 CO2 (waste) 12 H2O 36 ATP
(useful energy)
29
Lipids
Beta oxidation breaks down fatty acids to form
acetyl Coenzyme A. Lipids are more reduced (have
fewer oxygens) therefore, they have more
potential chemical energy and can be more fully
oxidized as an energy fuel. Therefore, we gain
more energy, gram for gram, from fats than from
carbohydrates.
30
Protein Metabolism
Amino acids may be deaminated and the resulting
carbon skeletons of whatever composition, can
be entered into the glycolytic or Krebs cycle
pathways to yield an energy harvest of ATPS. The
amino groups will be joined with CO2 molecules to
form the nitrogenous waste urea.
31
Nutrient Catabolism Pathways Are All
Interconnected
Ketone bodies result from excessive lipolysis and
fat catabolism a symptom of diabetes mellitus
32
The Daily Metabolic Cycle
- The body shifts back and forth physiologically
between the absorptive state and the
postabsorptive state. - The absorptive state occurs for approximately 4
hours after each regular meal. - The postabsorptive state takes over until the
next meal can be absorbed.
33
The Absorptive State
Insulin Dominates the Absorptive State All Cells
Rely on Glucose from the Meal for Energy
34
The Post-Absorptive State
Glucagon dominates the post-absorptive state and
is assisted by the insulin antagonists
(glucocorticoids, thyroid hormones, epinephrine,
and hGH).
Only Brain and Spinal Cord Cells Rely on Glucose
for Energy Most Other Body Cells Rely on Fatty
Acids for Energy.
35
The Post-Absorptive State
Glycogenolysis provides glucose fuel for skeletal
muscle. Glycogenolysis and gluconeogenesis in
the liver provide plasma glucose for nervous
tissue. Lipolysis supplies lipids to fuel all
other cells.
36
Lipid Transport by Lipoproteins
Lipoproteins transport hydrophobic lipids in a
droplet which is emulsified by an external layer
of phospholipids and proteins which make the
surface water soluble.
37
Lipid Transport by Lipoproteins
- Chylomicrons carry absorbed fat from the meal to
adipose tissue via lymph - VLDL and LDL carry cholesterol synthesized in the
liver and fat stored in the liver to body tissues - HDL carries excess cholesterol back to the liver
for catabolism and excretion - Increased total cholesterol and increased LDL are
linked to vessel and heart disease
38
Thermogenesis
39
Heat Loss to the Environment
- radiation away of infrared radiation
- convection and conduction of heat to air or water
surrounding the body - evaporation from sweating and from ventilating
respiratory membranes - vasodilation of cutaneous capillary beds
- decreased hormonal activity leading to decreased
basal metabolic rate (BMR) - behavioral stop exercising move to the shade,
take off clothes, turn on a/c, etc.
40
Heat Production/Conservation
- increased hormone activity (thyroxine,
epinephrine) leading to increased (BMR) - increased sympathetic ANS activity leading to
increased (BMR) - shivering of skeletal muscles
- vasoconstriction of dermal capillary beds
- behavioral start exercising, huddle together,
use clothing and shelter, use fire or other means
of heating the surroundings
41
Thermogenesis (cont.)
- A complex regulation involving negative feedback
control through endocrine and autonomic pathways
42
Pathology of Temperature Control
- fever
- heat cramps, heat exhaustion, heat stroke
- heat-induced dehydration
- burns
- hypothermia
- alcohol-induced hypothermia
43
End Chapter 24