Sn - PowerPoint PPT Presentation
Title: Sn
1
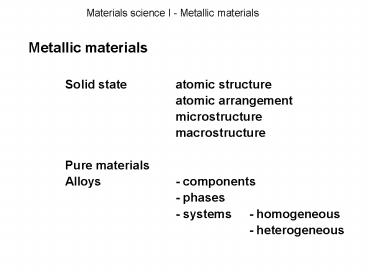
Materials science I - Metallic materials
Metallic materials Solid state atomic
structure atomic arrangement microstructur
e macrostructure Pure materials Alloys -
components - phases - systems -
homogeneous - heterogeneous
2
Materials science I - Metallic materials
- Solid state
- Atomic structure forces between atoms are
strong, changes of volume and shape needed
high energy. - Bonding of atoms in metallic materials is
METALLIC - electrons on last orbital of atoms are released,
atoms becomes positive ions. Free electrons are
sheared as the cloud or sea with other ions. - Metallic bond is equally strong in all
directions - Compounds with metallic bonds form a regular
lattice structures. - The free electrons can move materials with
metallic bonds are electrical conductive
3
Materials science I - Metallic materials
Atomic structures Crystalline - regular atom
distribution form the lattice (long distance
regularity) Amorphous - irregular atom
distribution (short distance regularity)
Metallic metals are mostly formed in 3
lattices FCC - face centred cubic K8 BCC -
body centred cubic K12 HCP - hexagonal close -
packed H12
4
(No Transcript)
5
Materials science I - Metallic materials
Microstructure Monocrystalline whole bulk has
the same orientation of lattice Ideal
monocrystalline is free of the lattice
imperfections Point faults (vacancies,
interstitial atoms, substitution atoms) Line
faults (dislocations edge, screw and general)
Areic faults (stacking faults, free surfaces,
grain boundaries) Volume faults (coherent
particles)
6
(No Transcript)
7
Polycrystalline in bulk of material exists
areas or volumes with different lattice
orientation called grains. Places, where grains
are connected are grain boundaries - narrow
areas (about 5 atoms) where the mismatch of
connecting lattices are compensed by suitable
distributed imperfections - atoms, vacancies and
dislocations.
8
Materials science I - Metallic materials
Microstructure Small structure components
visible only using the suitable type of
microscope atoms, dislocations, grains, grain
boundaries, secondary phase particles
(precipitate), impurities, number of presented
phases, their distribution, portion In
dimensions about nm and bigger
Macrostructure Visible by eye or with help of
magnifying glass. Large structure components as
big or dendritic grains (after casting, or some
heat treatment), impurities, In dimensions
about tens of mm and bigger.
9
Materials science I - Metallic materials
Pure metals Ideal pure metals are composed only
with the same atoms. Real material content
impurities becoming from their manufacturing.
Pure metals are marked as 99,9 . Very pure
materials content 99,999 of matrix atoms
Alloys Are solid state multicomponent systems
containing except atoms of fundamental metal the
added alloying elements and elements of
impurities more.
10
Materials science I - Metallic materials
Alloys Terms Components alloying
elements Phase homogeneous portion of a
system that has uniform physical and chemical
characteristics e.g. polymorphic forms in solid
state Single phase systems are termed
homogeneous e.g. melts, pure metals Multiphase
(two or more phases) are heterogeneous. Most of
the metallic alloys are heterogeneous systems.
11
Materials science I - Metallic materials
Solubility Solubility is a maximum concentration
of solute atoms in solid solution. Solid
solution Solid solution is formed in host
material latice, where the atoms of impurities
are solved. Atoms, which are smaller than atoms
of the host material forms interstitial solid
solution. Atoms fill the volumes between atoms of
the host material lattice (carbon in iron). Atoms
with close diameter (difference les 15 )
replaces some atoms in host materials. This type
of solid solution is substitutional (cooper and
nickel).
12
Materials science I - Metallic materials
Solid solutions Solubility unlimited the
atoms are mixed in whole range of
concentrations (from 0 to 100 )
limited exist some maximum concentration of
atoms which can be solved in host material.
Generally it is connected with temperature.
Interstitial solid solutions have in every
cases limited solubility (max 10 ) due to
limited free interstitial volume in lattice,
where the solute atoms can be placed.
13
Materials science I - Metallic materials
Equilibrium Equilibrium between phases in the
system is described with the Gibbs free energy G
H TS. The temperature and pressure play
role. Every change in system is described by
change of free energy ?G The system is in
equilibrium state, when the free energy reaches
minimum value. This state is termed stable. Each
change in system may lead to changes in
equilibrium. In real alloys, in solid state, if
the changes needs more time, the equilibrium
state is not reached. System will decrease free
energy during the time. The equilibrium is termed
metastable.
14
Materials science I - Metallic materials
Gibbs phase rule Is the criterion of number of
phases, which will coexist in equilibrium
conditions in the system P F C N P -
number of present phases F - number of degrees
of freedom (temperature, pressure,
composition etc.) C - number of components
(elements or stable compound of phase
diagrams) N - number of noncompositional
variables (temperature, pressure)
15
Materials science I - Metallic materials
Gibbs phase rule We search possible number of
freedom F C N - P Generally N 2 (if the
temperature and pressure play role) (equilibrium
in system with gaseous, liquid and solid
state) When we consider no pressure influence,
the N 1 (equilibrium phase diagrams of
alloys) For pure metal is C 1 ? exist only
one temperature, when coexist melt and solid
state solidification (crystallization) proceed
at constant temperature
16
Materials science I - Metallic materials
Cooling curves pure metal alloy
17
Materials science I - Metallic materials
Diffusion Phenomenon of material transport by
atomic motion in solid state only one possible.
Phase changes in the solid state systems are
mostly possible due to diffusion. In pure
materials the atoms changes their positions,
there are no changes in chemical composition -
self diffusion. In real alloy, the moving of
atoms lead to chemical content changes. This
phenomenon is termed as interdiffusion or
impurity diffusion.
18
Materials science I - Metallic materials
Diffusion
Difusion couple Cu - Ni
19
Materials science I - Metallic materials
Mechanisms of diffusion For atom moving into the
lattice, there must be an empty place, and the
atom must break the attraction forces their
neighbours. Vacancy mechanism moving own or
substitutional atoms to position of existing
vacancies. Interstitial diffusion moving
interstitial atoms into neighbour interstitionals
positions in lattice. The possible but low
frequented are exchange and ring mechanisms.
20
Materials science I - Metallic materials
Diffusion The moving atoms from their positions
is influenced by vibration in lattice. If the
amplitude of vibrations reach the value when the
barrier of atomic attraction forces is exceeded,
the atom can change your position. The energy
needed for amplitude increasing is called
activation energy Qd. Diffusion rate of
steady state diffusion is described by 1st Ficks
law J - diffusion flux D - diffusion
coefficient C - concentration x - position D0 -
temperature independent diffusion constant
21
Materials science I - Metallic materials
Diffusion The term is concentration gradient
of concentration profile Driving force of
the steady state diffusion is concentration
gradient Nonsteady state diffusion The time t of
the process is considered 2nd Ficks
law Solution is erf is the Gausian error
function
22
Materials science I - Metallic materials
Diffusion The depth of diffusion can be
expressed as h (Dt)1/2 or from equation for
middle quadratic distance of moving atoms
Volume or bulk diffusions is relative slow
process The diffusion is accelerated by lattice
imperfections. The other diffusion paths are
along dislocation, through the grain boundaries
or along free external surfaces. Temperature
accelerates diffusion but is not the driving
force! increases diffusion coefficient, increase
the atom vibration amplitude, increase the number
of vacancies.
23
Materials science I - Metallic materials
Solidification Solidification is process of
changing atomic coupling. The liquid state of
metal melt transforms to a solid state. The
system is driven to minimize the free energy.
?G GL - GS If the temperature
decreases, the equilibrium between liquid and
solid state is violate, and nuclei of solid state
(phase) can rise.
24
Materials science I - Metallic materials
Solidification Nucleation Consider formation
of small spherical nucleus with radius r, ? is
the surface energy of nucleus interface
Surface free energy
Critical nucleus size r is the minimum size of
nucleus, which is able further growth. ?G is the
nucleation barier
Volume free enrgy
25
Materials science I - Metallic materials
Solidification Nucleation homogeneous
fluctuation of energy, spontaneously heterogeneo
us on existing surfaces
Number of nuclei s is the number of available
nucleation sites
Rate of nucleation q is activation energy for
diffusion
26
Materials science I - Metallic materials
Solidification Nucleation homogeneous
fluctuation of energy, spontaneously heterogeneo
us on existing surfaces
27
Materials science I - Metallic materials
Growth of new phase Planar the growth is
controlled by heat removing through the solid
phase Dendritic heat removing is possible
through the solid phase and through the
melt.
28
Materials science I - Metallic materials
Casting structure Chill zone - first and fast
cooled part of ingot - near to surface
Columnar zone - area of long
crystals Eqiuaxed zone in the middle of the
ingot
29
Materials science I - Metallic materials
Eqilibrum phase diagram
30
Materials science I - Metallic materials
Eqilibrum phase diagram Lever rule
Weight of solid solution of composition m
bm Weight of liquid of composition q
qb Ratio bm/pb
31
(No Transcript)
32
Materials science I - Metallic materials
Eqilibrum phase diagram Structure development
33
Materials science I - Metallic materials
Eqilibrum phase diagram With eutectic reaction
34
Materials science I - Metallic materials
Eqilibrum phase diagram Hypoeutectic alloy
35
Materials science I - Metallic materials
Eqilibrum phase diagram Hypoeutectic alloy
36
Materials science I - Metallic materials
Eqilibrum phase diagram Hypoeutectic alloy
37
Materials science I - Metallic materials
Eqilibrum phase diagram eutectic alloy
38
(No Transcript)
39
(No Transcript)
40
(No Transcript)
41
(No Transcript)