Which of the following biomolecules consists of monomers of amino acids:
1 / 13
Title: Which of the following biomolecules consists of monomers of amino acids:
1
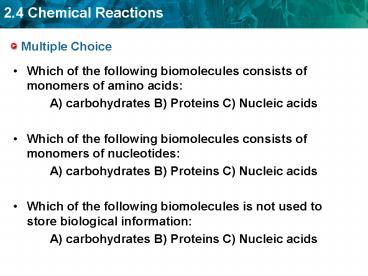
Multiple Choice
- Which of the following biomolecules consists of
monomers of amino acids - A) carbohydrates B) Proteins C) Nucleic acids
- Which of the following biomolecules consists of
monomers of nucleotides - A) carbohydrates B) Proteins C) Nucleic acids
- Which of the following biomolecules is not used
to store biological information - A) carbohydrates B) Proteins C) Nucleic acids
2
LEQ How do enzymes help cells perform chemical
reactions?
- Reading 2.5, 12.3 Unit 2 Test mon
- Activator Reaction Acrostic
- Term, phrase, idea using R-E-A-C-T-I-O-N
- Key terms bond energy, activation energy,
enzyme,
3
Bonds break and form during chemical reactions.
- Chemical reactions change chemicals into
different ones - breaking old bonds
- forming new bonds
O2 C6H12O6 CO2 H2O
Reactants Products
- Bond energy
- Energy is added to break bonds.
- released when bonds form.
4
Organisms require energy for a cell to maintain
homeostasis.
- Products must be generated
- Reversible reactions can reach equilibrium -
generally fatal to cells (no way to generate
products)
CO2 H2O H2CO3
5
Chemical reactions release or absorb energy.
- Activation energy needs to be absorbed to start a
chemical reaction. - Significantly delay rate of reaction
6
- Exothermic reactions release more energy than
they absorb.
- Products have lower bond energies
7
- Endothermic reactions absorb more energy than
they release.
- Products have higher bond energies.
8
A catalyst lowers activation energy.
- Catalysts are substances that speed up chemical
reactions. - decrease activation energy increase reaction rate
9
Enzymes are proteins that catalyze chemical
reactions without being consumed.
- Enzyme terms
- Substrate
- Reactant(s)
- Active site
- Binds substrates
- Substrate specific
10
- The lock-and-key model helps illustrate how
enzymes function.
11
Questions
- Chemical reactions tend to reach equilibrium when
they are left to proceed on their own. Why is
this dangerous for a cell (Hint how will this
affect the production of biomolecules)? - Biological catalysts are essential for cells to
function as systems. Do you think cells could
produce polymers by using uncatalyzed reactions?
Why or why not? - Consider exothermic and endothermic reactions.
How do cells find the energy to perform an
endothermic reaction?
12
Summary
- How is the structure of an enzymes binding site
related to its function? - When a chemical reaction reaches equilibrium
describe what is happening to reactants and
products. - What prevents a chemical reaction from occurring
spontaneously? - Speculate why has evolution produced nothing but
cells that specialize in catalyzed chemical
reactions? - How do catalyzed chemical reactions aid in
homeostasis? - How can exothermic and endothermic reactions be
considered a system? - The process below is exothermic. What must be
true about the bond energies of the reactants and
the products? Explain - 6O2 C6H12O6 ? 6CO2 6H2O
13
Answers
- Catalysts reduce the activation energy required
to start a chemical reaction - easier to
rearrange chemical bonds to form product. - Enzymes bring substrates close together so that
they can react and slightly alter the bonds
within the substrates by changing the shapes of
the molecules (lowers the activation energy
barrier). - Endothermic reactions absorb energy and their
products have higher bond energy (energy goes
into bond formation). Exothermic reactions
release energy and their products have lower bond
energy (energy leaves the reaction site when
bonds break). - Bond energies must be higher in reactants than
those of products energy is released