Overview - PowerPoint PPT Presentation
1 / 1
Title:
Overview
Description:
Disaccharide Isomers Differentiated with Infrared Multiple Photon Dissociation Sarah E. Stefan, John R. Eyler Department of Chemistry, University of Florida ... – PowerPoint PPT presentation
Number of Views:44
Avg rating:3.0/5.0
Title: Overview
1
Disaccharide Isomers Differentiated with Infrared
Multiple Photon Dissociation Sarah E. Stefan,
John R. Eyler Department of Chemistry,
University of Florida, Gainesville, FL USA
Results
Results Continued
Overview
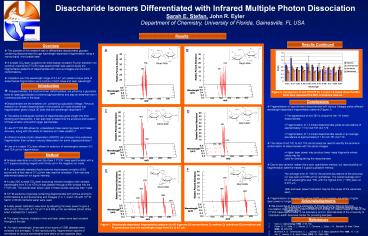
A
B
- The purpose of this research was to
differentiate deprotonated glucose-containing
disaccharides through wavelength-dependent
fragmentation using a narrow-band, line-tunable
laser. - A tunable CO2 laser coupled to an electrospray
ionization Fourier transform ion cyclotron
resonance (FTICR) mass spectrometer was used to
study the fragmentation patterns of disaccharides
with various linkages and anomeric conformations.
- Irradiation over the wavelength range of 9.2-9.7
µm yielded unique plots of disaccharide
fragmentation as a function of both mass and
laser wavelength.
Glcß1-3Glc
Glca1-3Glc
Ratio
Introduction
- Polysaccharides, the most common carbohydrates,
are joined by a glycosidic bond to lipids
(glycolipids) or proteins (glycoproteins) and
play an essential role in numerous activities in
the body. - Disaccharides are the smallest unit containing a
glycosidic linkage. Previous research on
lithiated disaccharides in the positive ion mode
showed that fragmentation gives unique 2D plots
that are wavelength dependent. 2 - The ability to distinguish isomers of
disaccharides gives insight into their bonding
and interactions. It can also help tp determine
the structure and location of these smaller units
within larger saccharides. - Use of FTICR-MS allows for unparalleled mass
resolving power and mass accuracy, along with the
ability for selective ion mass isolation.3 - Infrared multiple photon dissociation (IRMPD)
can produce more extensive fragmentation than
collision induced dissociation for some
oligosaccharides.4 - Use of a tunable CO2 laser allows for selection
of wavelengths between 9.2 and 10.8 µm for
fragmentation.
Figure 2. Comparison of m/z 161/179 for 1-3 and
1-6 linked disaccharides.
Error bars represent the 95 confidence interval.
Conclusions
- Fragmentation of deprotonated disaccharides with
various linkages yields different
wavelength-dependent fragmentation patterns
(Figure 1). - The appearance of m/z 281 is unique for the 1-6
linked disaccharides. - Fragmentation of 1-3 linked disaccharides yields
an abundance of approximately 11 for m/z 161m/z
179. - Fragmentation of 1-4 linked disaccharides
results in an average abundance of approximately
61 for m/z 161m/z 179. - The ratios of m/z 161 to m/z 179 cannot solely
be used to identify the anomeric conformation of
disaccharides with the same linkages. - Higher laser power may produce lower mass
fragments whose ratios may be - used for distinguishing the disaccharides
- Day-to-day variation makes this a poor
quantitative method, but reproducibility of
fragmentation patterns makes it a good
qualitative method. - An average error of 3 for the percent abundance
of the precursor ion was seen at 9.588 µm for
isomaltose. The overall average error for all
wavelengths was 6, with the highest error
(19) seen at 9.201 µm. - ESI and laser power fluctuation may be the cause
of the variances seen.
C
D
Glcß1-4Glc
Glca1-4Glc
Method
- Analysis was done on a Bruker Bio-Apex II FTICR
mass spectrometer with a 4.7T superconducting
magnet and Infinity cell in the negative ion
mode. - A pneumatically-assisted Apollo external
electrospray ionization (ESI) source with a flow
rate of 3-7 µL/min was used for ionization. Flow
rate was determined based on ion signal
intensity. - A Lasy-20G tunable CO2 laser producing infrared
irradiation with variable wavelengths from 9.2 to
10.8 µm was passed through a KBr window into the
FTICR cell. The typical laser power used in these
studies was less than 1 watt. - 10-4 M solutions of glucose-containing
disaccharides with various anomeric conformations
(a-and ß-anomers) and linkages (1-3, 1-4 and 1-6)
with 10-3 M NaOH in 8020 methanolwater were
used. - A daily power calibration was done by adjusting
the laser power to give a ratio of m/z 179 to 341
of 1.19 0.17 at 9.588 µm for isomaltose
(Glca1-6Glc) when irradiated for 1 second. - The signal intensity, irradiation time and laser
power were kept constant throughout the day. - For each wavelength, three sets of ten scans of
128K datasets were collected and averaged. To
test reproducibility, fragmentation spectra of
isomaltose at various wavelengths were taken on
two separate days.
F
E
Glcß1-6Glc
Glca1-6Glc
Acknowledgements
- We would like to thank the University of Florida
and the National Science Foundation (NSF grant
No. CHE-0718007) for funding, Dr. David Powell
for use of the FTICR mass spectrometer in his
laboratory and Dr. Brad Bendiak of the University
of Colorado Health Sciences Center for providing
samples.
References
- Varki, A. Glycobiology 1993, 3, 97-130.
- Polfer, N. C. Valle, J. J. Moore, D. T.
Oomens, J. Eyler, J. R. Bendiak, B. Anal. Chem.
2006, 78, 670-679. - Marshall, A. G. Hendrickson, C. L. Jackson, G.
S. Mass Spectrom Rev 1998, 17, 1-35. - Xie, Y. Lebrilla, C. B. Anal. Chem. 2003, 75,
1590-1598.
Figure 1. Wavelength-dependent fragmentation
patterns for A) nigerose B) laminaribiose C)
maltose D) cellobiose E) isomaltose and
F) gentiobiose over the wavelength
range from 9.2 to 9.7 µm.