BBC - PowerPoint PPT Presentation
BBC
Stoichiometry Vocab Theoretical Yield: the calculated amount of product yielded by a reaction (found through stoichiometry) Actual Yield: the actual amount of product ... – PowerPoint PPT presentation
Title: BBC
1
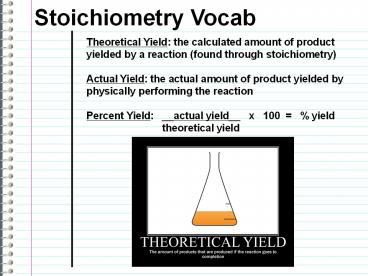
Stoichiometry Vocab
Theoretical Yield the calculated amount of
product yielded by a reaction (found through
stoichiometry) Actual Yield the actual amount
of product yielded by physically performing the
reaction Percent Yield __actual yield__ x
100 yield
theoretical yield
2
Stoichiometry Vocab
Molar Mass the mass obtained by adding up the
weights of all the atoms in a compound Given
the value (a number) that is given in a
stoichiometry problem look for a number and then
moles or grams ex 3.0 moles of CH4 or 23.0
g of NaCl Wanted the quantity that the problem
is asking you to find look for How many or
Calculate ex How many grams of CH4 are
produced Calculate the moles of NaCl
required
3
Stoichiometry Vocab
Limiting Reactant (Limiting Reagent) the
reactant that limits how much product you can
make can change depending on how much of each
reactant you have Excess/Sufficient
usually refers to reactants not a factor in
stoichiometry since it means you have more than
enough and it will not be a limiting
reactant Mole Ratio (Molar Ratio) ratio of any
substances in a balanced equation used to
convert one quantity in a reaction to another
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.








![⚡Read✔[PDF] The Beatles: The BBC Archives PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10071316.th0.jpg?_=202407030812)



![⚡[PDF]✔ Lord Peter Wimsey: BBC Radio Drama Collection, Volume 3: Four BBC Radio 4 PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10062886.th0.jpg?_=20240624075)





![book❤️[READ]✔️ Writers on Walks: A BBC Radio 3 Collection: 30 Reflections from E PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10132206.th0.jpg?_=20240917107)
![book❤️[READ]✔️ Writers on Walks: A BBC Radio 3 Collection: 30 Reflections from E PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10136173.th0.jpg?_=202409231212)
![❤[PDF]⚡ C. S. Lewis at the BBC: Messages of Hope in the Darkness of War PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10072933.th0.jpg?_=20240704114)
![book❤️[READ]✔️ Writers on Walks: A BBC Radio 3 Collection: 30 Reflections from Exploring on Foot PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10134204.th0.jpg?_=20240920054)
![[PDF] Raymond Chandler: The BBC Radio Drama Collection Android PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10089798.th0.jpg?_=202408011210)





![[PDF] London Calling: Britain, the BBC World Service and the Cold War Ipad PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10089691.th0.jpg?_=20240731126)

