Chapter 5 - PowerPoint PPT Presentation
Title:
Chapter 5
Description:
Title: No Slide Title Author: Youngstown State University Last modified by: Peter Norris Created Date: 8/30/2000 5:06:32 PM Document presentation format – PowerPoint PPT presentation
Number of Views:62
Avg rating:3.0/5.0
Title: Chapter 5
1
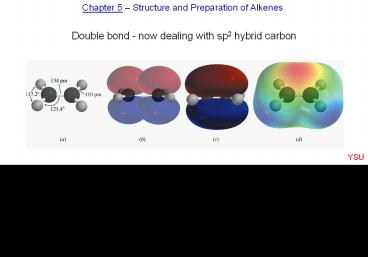
Chapter 5 Structure and Preparation of Alkenes
Double bond - now dealing with sp2 hybrid carbon
YSU
2
5.1 Structure and Nomenclature of Alkenes
YSU
1-butene
1-hexene
2-methyl-2-hexene
6-bromo-3-propyl-1-hexene
2,3-dimethyl-2-butene
5-methyl-4-hexen-1-ol
3
Common Alkene Substituents
YSU
vinyl
allyl
isopropenyl
Cycloalkenes
cyclohexene
3-bromocyclooctene
1-chlorocyclopentene
4
5.2 Structure and bonding in ethylene Figure 5.1
YSU
5
5.3-5.4 cis-trans isomerism in alkenes
1-butene
2-methylpropene
cis-2-butene
trans-2-butene
Cinnamaldehyde (trans alkene - E)
cis alkene (Z)
See Table 5.1 for priority rules
YSU
6
Interconversion of cis and trans-2-butene
YSU
7
5.5-5.6 Heats of combustion of isomeric C4H8
alkenes
Figure 5.3
YSU
8
5.5-5.6 Heats of combustion of isomeric C4H8
alkenes Figure 5.2
Generally, the more substituted an alkene, the
more stable
YSU
9
Molecular models of cis-2-butene and
trans-2-butene
Figure 5.4
YSU
10
5.7 Cycloalkenes - trans not necessarily more
stable than cis
C-12 cis and trans equal in energy
Sterculic acid (natural product)
YSU
11
5.8 Preparation of Alkenes - Elimination reactions
YSU
5.9 Dehydration of Alcohols
12
5.10 Zaitsev Rule
Dehydration usually results in more highly
substituted alkene being major product - Zaitsev
rule (regioselectivity)
YSU
13
5.10 Zaitsev Rule
YSU
14
5.11 Stereoselectivity in Alcohol Dehydration
One stereoisomer is usually favoured in
dehydrations
When cis and trans isomers are possible in this
reaction the more stable isomer is usually formed
in higher yield
YSU
15
5.12 Acid-catalyzed Alcohol Dehydration E1
E1
YSU
16
5.13 Carbocation Rearrangements in E1 Reactions
Cation rearrangement leads to more stable cation
YSU
17
Orbital representation of methyl migration
Figure 5.6
YSU
18
5.13 Hydride shifts to more stable carbocations
YSU
1o carbocation?????
19
5.14 Dehydrohalogenation - Elimination with loss
of H-X
YSU
100
Zaitsev rule followed for regioisomers when a
small base such as NaOCH3, NaOCH2CH3 is used.
Trans usually favoured over cis.
20
5.15 The E2 Mechanism - Elimination Bimolecular
- Reaction occurs under basic conditions
- Reaction is concerted
- Rate depends on basealkyl halide i.e.
Bimolecular - E2 - C-H bond breaking, CC bond forming and C-X
bond breaking - events all occur at the same time
YSU
21
The E2 Mechanism - Elimination Bimolecular
YSU
22
5.16 Anti Elimination faster than Syn Elimination
YSU
E2 Elimination usually faster when H and leaving
group are anti periplanar as opposed to syn
periplanar.
23
Conformations of cis- and trans-4-tert-butylcycloh
exyl
YSU
24
Favourable conformations for fast elimination
E2 Elimination usually faster when H and leaving
group are anti periplanar as opposed to syn
periplanar.
YSU
25
Not covering Section 5.17 (Isotope Effects)
YSU
26
5.18 Different Halide Elimination Mechanism - E1
R.D.S. is now unimolecular, E1 - usually under
neutral/acidic conditions
YSU