Matter PowerPoint PPT Presentation
Title: Matter
1
Matter
2
Everything that has mass and volume is called
matter.
What is matter?
3
The Nature of Matter
Gold
Mercury
- Chemists are interested in the nature of matter
and how this is related to its atoms and
molecules.
4
Page 2
5
Matter Flowchart
MATTER
yes
no
Can it be physically separated?
Homogeneous Mixture (solution)
Heterogeneous Mixture
Compound
Element
6
Types of Mixtures
- Variable combination of 2 or more pure substances.
Heterogeneous visibly separate phases
Homogeneous Same throughout
7
Page 3
Page 5
Homework Pages 6 and 7
8
Page 10 11Homework 13 14
9
Kinetic Nature of Matter
- Matter consists of atoms and molecules in _____.
10
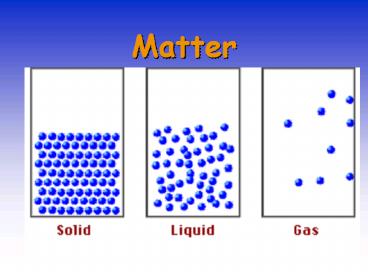
STATES OF MATTER
- _______ have rigid shape, fixed volume.
External shape can reflect the atomic and
molecular arrangement. - Reasonably well understood.
- _______ have no fixed shape and may not fill a
container completely. - Not well understood.
- _______ expand to fill their container.
- Good theoretical understanding.
11
Physical Properties
- What are some physical properties?
- color
- melting and boiling point
- odor
12
- Graphite layer structure of carbon atoms
reflects physical properties.
13
Physical Changes
- can be observed without changing the identity of
the substance - Some physical changes would be
- boiling of a liquid
- melting of a solid
- dissolving a solid in a liquid to give a
homogeneous mixture a SOLUTION.
14
Chemical Properties and Chemical Change
- Burning hydrogen (H2) in oxygen (O2) gives H2O.
15
Chemical Properties and Chemical Change
- Burning hydrogen (H2) in oxygen (O2) gives H2O.
- Chemical change or chemical reaction
transformation of one or more atoms or molecules
into one or more different molecules.
16
Sure Signs of a Chemical Change
- Heat
- Light
- Gas Produced (not from boiling!)
- Precipitate a solid formed by mixing two
liquids together
17
Physical vs. Chemical
- physical
- chemical
- physical
- physical
- chemical
- Examples
- melting point
- flammable
- density
- magnetic
- tarnishes in air
18
Physical vs. Chemical
- Examples
- rusting iron
- dissolving in water
- burning a log
- melting ice
- grinding spices
19
- Page 15
- Homework page 16
20
- How do we separate a mixture?
- Differences in properties such as
- density
- particle size
- molecular polarity
- boiling point and freezing point
- Solubility
- These differences permit physical separation
21
Separation techniques
- Filtration
- Solubility
- Distillation
- Boiling Point
- Chromatography
- Density and Polarity
21
22
Density and polarity
Particle size
Boiling point
23
- Page 25 26
- Homework page 27