Conc Acid group - PowerPoint PPT Presentation
1 / 19
Title:
Conc Acid group
Description:
Part 2 To 1ml of the solution add1ml of saturated ammonium sulphate solution followed by 0.1 g sodium thiosulphate. Heat on water bath for 5 minutes. – PowerPoint PPT presentation
Number of Views:69
Avg rating:3.0/5.0
Title: Conc Acid group
1
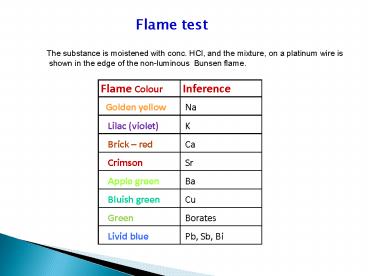
Flame test
The substance is moistened with conc. HCl, and
the mixture, on a platinum wire is shown in the
edge of the non-luminous Bunsen flame.
Flame Colour Inference
Golden yellow Na
Lilac (violet) K
Brick red Ca
Crimson Sr
Apple green Ba
Bluish green Cu
Green Borates
Livid blue Pb, Sb, Bi
2
Dil. Acid group
To about 5 mg of the substance add about 0.5 ml
of dil. HCl. Observe the reaction in the cold
and then heat it on a water bath.
Observation Inference
1. Brisk effervesce, the gas turns lime/Baryta water milky Carbonate is Present.
2. Colourless gas (SO2) with the smell of burning sulphur .The gas turns a filter paper dipped in acidified potassium dichromate green. Sulphite is Present.
3. Colourless gas with the odour of rotten eggs (H2S) is evolved. The gas turns lead acetate paper black and cadmium acetate paper yellow. Sulphide is Present.
4. Vineger smell. Red coloration/ppt with neutral ferric chloride. 5. Mix 20 mg of the substance with 1ml of ethyl alcohol and 5 drops of con. H2SO4, Heat in a hot water rack for 10 minutes, and pour into 2 ml of Na2CO3 solution. ? Fruity odour Acetate is Present.
3
Conc. Acid group
Addition of Conc. H 2SO4 MnO2, heat
Observation Inference
1. Greenish yellow pungent smelling gas (HCl) which fumes in moist air. Dense white fumes (NH4Cl) with a drop of ammonia on a glass rod Chloride is Present.
2. Reddish brown fumes (Br2) are evolved. Bromide is Present.
3. violet vapours (I2) are evolved. Iodide is Present.
4.On warming, brown gas (NO2)With characteristic smell is evolved. The brown colour is deepened by the addition of copper turnings. Nitrate is Present.
4
Silver nitrate group Add silver nitrate to
Neutralized sodium carbonate extract
1 A curdy white precipitate (AgCl), insoluble in dil.HNO3, but soluble in ammonia solution. Chloride is confirmed.
2 A pale yellow precipitate (AgBr), insoluble in dil. HNO3, but sparingly soluble in ammonia solution. Bromide is confirmed.
3 A yellow precipitate, insoluble in both dil. HNO3 and ammonium solution. Iodide is confirmed.
5
Test for Bromide and Iodide To the substance in
dil. HNO3 add drops of KMnO4 solution until the
pink colour persists. Add CCl4 and shake.
Reddish brown colouration of CCl4 layer Bromide is confirmed.
Violet colouration of CCl4 layer. Iodide is confirmed.
6
Nitrate Brown ring test The sodium carbonate
extract is acidified with dil. H2SO4. An equal
volume of freshly prepared FeSO4 solution is
added. Holding the test-tube in an inclined
position con. H2SO4 drops are added without
shaking.
A brown ring is formed at the junction of the two layers Nitrate is confirmed.
7
- Sulphate BaCl2 test
- To the sodium carbonate extract add dil. HCl till
no more CO2 is evolved. Add 1-2 ml of dil. HCl
and BaCl2 solution.
A white precipitate insoluble in dil. HCl is formed Sulphate is Confirmed
Borate Flame Test The substance is mixed with
calcium flouride and con. H2SO4 to get a paste.
Hold some of the paste on a platinum loop, just
outside the base of the Bunsen flame.
A green flame is formed Borate is confirmed.
8
Phosphate Amm. Molybdate test To the sodium
carbonate extract add dil. HNO 3ill no more CO2
is evolved. Add 1-2 ml of amm. Molybdate. Warm.
Yellow ppt Phosphate is confirmed.
Oxalate
- Acidify the sodium carbonate extract with
dilute acetic acid and add calcium chloride
solution. White ppt. - Divide the precipitate into two parts
- Add dilute HCl ? ppt dissolves
- Add hot dil. H2 SO4 and potassium permanganate
drop wise ? it gets decolourised - Oxalate is confirmed
9
- Analysis of Cations
- Preparation of the original solution
- A small quantity of the substance (15 mg) is
treated with the following solvents in the given
order. - Distilled water
- dil.HCl,
- dil.HNO3
- con.HCl
- aqua regia (3 vol. con. HCl 1 vol. con. HNO3).
- Observe the solubility in the cold, then heat to
boiling. If any gases are formed, boil them off.
Dissolve 50 to 100 mg of the substance in the
suitable solvent and prepare the solution. This
solution is often referred to as the original
solution.
10
Cations/ Groups / Group reagents
Group Group reagent Cations Ppt formed
I Dil. HCl Pb2 , Ag 1 , Hg1 Chlorides
II Dil. HCl H S Pb2 , Bi3 , Cd2 , Cu2 , Sn2 , As3 , Sb3 Sulphides
III NH Cl NH OH Fe3 , Al3 , Cr3 Hydroxides
IV NH Cl NH OH H S Co2 , Ni2 , Zn2 , Mn2 Sulphides
V NH Cl NH OH NH CO Ca2 , Ba2 , Sr2 Carbonates
VI No group reagent Mg2 , NH , K --
11
Separation of Cations into Groups
To 1 ml of the original solution in a centrifuge tube, dil. HCl is added until precipitation, if any, is complete Centrifuge. To 1 ml of the original solution in a centrifuge tube, dil. HCl is added until precipitation, if any, is complete Centrifuge. To 1 ml of the original solution in a centrifuge tube, dil. HCl is added until precipitation, if any, is complete Centrifuge. To 1 ml of the original solution in a centrifuge tube, dil. HCl is added until precipitation, if any, is complete Centrifuge.
Residue-1 White, may contain PbCl2, Hg2Cl2 or AgCl. Group-1 present Centrifugate-1 Heat on a water bath pass H2S gas until the precipitation is complete. Centrifuge. Centrifugate-1 Heat on a water bath pass H2S gas until the precipitation is complete. Centrifuge. Centrifugate-1 Heat on a water bath pass H2S gas until the precipitation is complete. Centrifuge.
Residue-1 White, may contain PbCl2, Hg2Cl2 or AgCl. Group-1 present Residue-2 May Contain BlackHgS, PbS, CuS. BrownBi2S3. YellowCdS, Sb2S3,SnS2 Group 2 present Centrifugate-2 (Eliminate the interfering anions if necessary.Boil off H2S Add 3 drops of con.HNO3 and boil.Add 100 mg solid ammonium chloride, heat on a water bath. Add ammonia solution till alkaline, and add 2 drops excess. Warm. Stir. Centrifuge. Centrifugate-2 (Eliminate the interfering anions if necessary.Boil off H2S Add 3 drops of con.HNO3 and boil.Add 100 mg solid ammonium chloride, heat on a water bath. Add ammonia solution till alkaline, and add 2 drops excess. Warm. Stir. Centrifuge.
Residue-1 White, may contain PbCl2, Hg2Cl2 or AgCl. Group-1 present Residue-2 May Contain BlackHgS, PbS, CuS. BrownBi2S3. YellowCdS, Sb2S3,SnS2 Group 2 present Residue-3 May contain Reddish-brownFe(OH)3 GreenCr(OH)3 WhiteAl(OH)3 Group 3 Present Centrifugate-3 Add 2 drops of NH3 solution. Warm. Pass H2S to complete precipitation. Centrifuge. Wash the residue
12
Separation of Cations into Groups Contd.
Residue-4 May contain Black CoS, NiS Pink MnS, White ZnS Group 4 present Centrifugate-4 Place in a china dish. Acidify with dil. Acetic acid. Evaporate to a pasty mass. Add 5 drops of con.HNO3.Heat to dryness (till fumes stop)7 Dissolve the residue in 5 drops of dil. HCl and 1 ml water. Add (in test-tube) 5 drops of 20 NH4Cl. Add NH4OH with shaking till alkaline. Add excess of 10 (NH4)2CO3 soln. Warm at 50-60oC. Centrifuge. Wash. Centrifugate-4 Place in a china dish. Acidify with dil. Acetic acid. Evaporate to a pasty mass. Add 5 drops of con.HNO3.Heat to dryness (till fumes stop)7 Dissolve the residue in 5 drops of dil. HCl and 1 ml water. Add (in test-tube) 5 drops of 20 NH4Cl. Add NH4OH with shaking till alkaline. Add excess of 10 (NH4)2CO3 soln. Warm at 50-60oC. Centrifuge. Wash.
Residue-4 May contain Black CoS, NiS Pink MnS, White ZnS Group 4 present Residue -5 May contain White BaCO3,SrCO3, CaCO3 Group 5 present Centrifugate-5 Evaporate to a pasty mass, add 0.5 ml con.HNO3. Heat to dryness White residue- Group-6 present
13
Analysis of Group I
Separation of group 1 cations The Residue-1 is washed with cold water containing a few drops of dil. HCl, and centrifuged. To the residue, add 1 ml of hot water. Heat to boiling for 1-2 minutes. Centrifuge while hot. Transfer the centrifugate quickly to another test tube. Separation of group 1 cations The Residue-1 is washed with cold water containing a few drops of dil. HCl, and centrifuged. To the residue, add 1 ml of hot water. Heat to boiling for 1-2 minutes. Centrifuge while hot. Transfer the centrifugate quickly to another test tube. Separation of group 1 cations The Residue-1 is washed with cold water containing a few drops of dil. HCl, and centrifuged. To the residue, add 1 ml of hot water. Heat to boiling for 1-2 minutes. Centrifuge while hot. Transfer the centrifugate quickly to another test tube.
Residue (Residue 1.1) White May contain Hg2Cl2 and AgCl. Wash with boiling water to remove the undissolved PbCl2.Treat the residue with 0.5 ml warm dilute NH3 solution. Stir. Centrifuge. Residue (Residue 1.1) White May contain Hg2Cl2 and AgCl. Wash with boiling water to remove the undissolved PbCl2.Treat the residue with 0.5 ml warm dilute NH3 solution. Stir. Centrifuge. Centrifugate (1.1) May contain PbCl2. Divide into 3 parts. 1. Cool under tap White ppt. reappears . Pb2 is confirmed. 2. Add 2 drops of potassium chromate Yellow ppt. (PbCrO4).- Pb2 is confirmed. 3. Add 2 drops of KI solution Yellow ppt. (PbI2).Boil the ppt. with water and a few drops of acetic acid and cool. The ppt. dissolves on heating and reappears as golden spangles on cooling Pb2 is confirmed.
Residue (1.2), BlackHg Hg (NH2) Cl. Hg22 present. Dissolve the ppt. in aqua-regia, heat, divide into two parts. 1. Add stannous chloride - White grayish ppt. Hg22 is confirmed. 2. Add drops of KI solution- Red or Yellow ppt. Hg22 is confirmed. Centrifugate (1.2) May contain Ag (NH3)2Cl. Divide into 2 parts. 1. Add dil.HNO3. White ppt. (AgCl) Ag2 is confirmed. 2. Add KI solution- Yellow ppt. (AgI) Ag 2is confirmed. Centrifugate (1.1) May contain PbCl2. Divide into 3 parts. 1. Cool under tap White ppt. reappears . Pb2 is confirmed. 2. Add 2 drops of potassium chromate Yellow ppt. (PbCrO4).- Pb2 is confirmed. 3. Add 2 drops of KI solution Yellow ppt. (PbI2).Boil the ppt. with water and a few drops of acetic acid and cool. The ppt. dissolves on heating and reappears as golden spangles on cooling Pb2 is confirmed.
14
Analysis of Group II A
Residue-2A May contain Hg2, Pb2, - Bi 3 , Cu2 and Cd2 Add 1.5 ml dil.HNO3. Warm and centrifuge. Residue-2A May contain Hg2, Pb2, - Bi 3 , Cu2 and Cd2 Add 1.5 ml dil.HNO3. Warm and centrifuge. Residue-2A May contain Hg2, Pb2, - Bi 3 , Cu2 and Cd2 Add 1.5 ml dil.HNO3. Warm and centrifuge. Residue-2A May contain Hg2, Pb2, - Bi 3 , Cu2 and Cd2 Add 1.5 ml dil.HNO3. Warm and centrifuge.
Residue Black HgS, Wash with Water. Dissolve in 3 drops con. HCl and 1 drop con. HNO3. Heat. Divide into 2 parts. 1. Add 2 drops SnCl2.White grey precipitate. Hg2 is confirmed 2. Add KI solution Red precipitate. dissolving in excess KI. Hg2 is confirmed. Centrifugate- May contain nitrates of Pb, Bi, Cu, and Cd. Add excess con.NH3 solution and centrifuge. Centrifugate- May contain nitrates of Pb, Bi, Cu, and Cd. Add excess con.NH3 solution and centrifuge. Centrifugate- May contain nitrates of Pb, Bi, Cu, and Cd. Add excess con.NH3 solution and centrifuge.
Residue Black HgS, Wash with Water. Dissolve in 3 drops con. HCl and 1 drop con. HNO3. Heat. Divide into 2 parts. 1. Add 2 drops SnCl2.White grey precipitate. Hg2 is confirmed 2. Add KI solution Red precipitate. dissolving in excess KI. Hg2 is confirmed. Residue May contain Bi3 and Pb2. Add 1ml NaOH solution. Warm. Centrifuge. Residue May contain Bi3 and Pb2. Add 1ml NaOH solution. Warm. Centrifuge. Centrifugate May contain Cu2, and Cd2. 1. a) solution is colourless - Cu2 is absent. b) Pass H2S gas through the solution. Yellow precipitate Cd2 is confirmed. 2. a) Solution is blue Cu2 is present. Divide into 2 parts 1. Add acetic acid in excess and 1 drop K4 Fe (CN)6 solution. Red-brown precipitate. Cu2 is confirmed. 2. Add KCN (poison) to discharge blue colour. Pass H2S. Yellow precipitate. Cd2 is confirmed.
Residue Black HgS, Wash with Water. Dissolve in 3 drops con. HCl and 1 drop con. HNO3. Heat. Divide into 2 parts. 1. Add 2 drops SnCl2.White grey precipitate. Hg2 is confirmed 2. Add KI solution Red precipitate. dissolving in excess KI. Hg2 is confirmed. Residue May be Bi (OH)3 wash with water. Divide the precipitate into 2 parts. 1. Add sodium stannite reagent. Immediate blackening of precipitate. Bi3 is present. 2. Dissolve in 3 drops of Conc.HNO3. Pour 1 drop of the solution in 5 ml water- white turbidity- Bi 3 is confirmed. 3. Dilute the above solution. To one drop, on a spot plate, add a drop of Cinchonine KI reagent. Orange-red spot- Bi3 is confirmed. Centrifugate May contain Pb (OH)4-. Acidify with dil. Acetic acid. Divide into 2 parts. 1. Add K2CrO4 solution Yellow precipitate. Pb2 is confirmed. 2. Add dil.H2SO4 white precipitate. Pb2 is confirmed. Centrifugate May contain Cu2, and Cd2. 1. a) solution is colourless - Cu2 is absent. b) Pass H2S gas through the solution. Yellow precipitate Cd2 is confirmed. 2. a) Solution is blue Cu2 is present. Divide into 2 parts 1. Add acetic acid in excess and 1 drop K4 Fe (CN)6 solution. Red-brown precipitate. Cu2 is confirmed. 2. Add KCN (poison) to discharge blue colour. Pass H2S. Yellow precipitate. Cd2 is confirmed.
15
Analysis of Group II B
The centrifugate-2Amay contain Sb3 and Sn2 .
Centrifuge. Wash the Residue. Reject washings.
Treat the residue with 1ml conc. HCl. Warm on
water bath for 3 minutes. Stir. Centrifuge.
Residue- May contain HgS. Black It is tested for Hg as given in the Group 2 A. (Yellow precipitate. of As2O3 also appears here. Centrifugate- May contain HSbCl4 and H2SnCl6.Take small portions and test as under 1. Add NH3 solution till just alkaline. Add 0.3 gms of oxalic acid. Pass H2S for 30 seconds. Orange precipitate. (Sb2S3)-Sb 3 is present. 2. To 2 drops of the solution on a spot plate, add a minute crystal of NaNO2 Stir. Add 2 drops of Rhodamine-B reagent. Violet colouration- Sb 3 is present. 3. Treat 0.3ml of the solution with 10mg of Mg powder. Add 2 drops of FeCl3 solution, 3 drops of 5 tartaric acid solution, 2 drops of dimethyl glyoxime reagent, then dil.NH3 solution until basic. Red colouration. Sn4 is present.
16
ANALYSIS OF GROUP III The group III precipitate.
(Residue-3) may contain Fe3, Al3 and Cr3.
Dissolve the precipitate in 2ml of NaOH solution
in a boiling tube, add 1ml of 3 H2O2. Boil
gently and centrifuge.
Residue May Contain Fe3 .Dissolve the precipitate in 0.5ml of dil.HNO3. Add potassium ferrocyanide Deep blue precipitate.-Fe3 is present Confirmatory test for Fe2And Fe3 1. To the original solution add ammonium thiocyanate solution. a)No colouration.Fe2 is confirmed b)Deep red colouration. Fe3 is confirmed. 2. To the original solution Potassium ferrocyanide solution is added. a)Deep blue colour. Fe3 is confirmed. b)Pale blue colour. Fe2 is confirmed. Centrifugate May contain NaAlO2 (colourless), and NaCrO4 (yellow).(Test only for Al if the solution is colourless. Test portions as under. 1. Add dil. HCl till acidic. Add NH3 solution. till just alkaline, add 1 drop more. Warm. White Gelatinous precipitate of Al(OH)3. .Al3 is present. 2. Centrifuge the above precipitate. Dissolve in dil. HCl, add 0.3ml ammonium acetate solution and1 drop of aluminon reagent .A bright red precipitate. Al3 is confirmed. 3. To a drop of the centrifuge-3.1solution on a spot plate, add 1drop of 1 aqueous alizarin-S. Add drops of acetic acid until violet colour appears. Add a few more drops of acetic acid. A red precipitate or colouration appears. Al3 is confirmed. 4.Acidify with dil. acetic acid. Add 1 drop of lead acetate solution. Yellow precipitate.(PbCrO4).Cr3 present. 5. Acidify with dil. HNO3, cool, add 0.5ml of amyl alcohol or ether and 2 drops of 3 H2O2.Shake. Allow to stand. The organic layer becomes blue which is unstable. Cr3 is confirmed.
17
ANALYSIS OF GROUP IV The group IV precipitate
(Residue-4) may contain Co2, Ni2, Mn 2 and
Zn2. If not black, Co2 and Ni2 are absent.
Stir the precipitate in the cold, with very dil.
HCl. Centrifuge.
Residue May Contain Co2and Ni2. Dissolve the precipitate. in 5 drops of aquaregia. Divide into 3 parts. 1. Add amyl alcohol and 50mg solid NH4SCN. Shake. Blue colouration in the alcohol layer Co2 is confirmed. 2. Add drops of 1 alcoholic alpha-nitroso-betanaphthol. Reddish brownprecipitate. Co2 is confirmed. 3. Add 1 drop of NH4Cl solution. Make it faintly alkaline with NH3 solution. Add 3-5 drops of dimethyl glyoxime reagent. Redprecipitate. Ni2 is confirmed. Centrifugate May contain Mn2 and Zn2. Boil off to expel H2S Cool. Add excess of NaOH solution until basic and 4 drops of 3 H2O2 solution. Warm for 3 minutes. Centrifuge. Centrifugate May contain Mn2 and Zn2. Boil off to expel H2S Cool. Add excess of NaOH solution until basic and 4 drops of 3 H2O2 solution. Warm for 3 minutes. Centrifuge.
Residue May Contain Co2and Ni2. Dissolve the precipitate. in 5 drops of aquaregia. Divide into 3 parts. 1. Add amyl alcohol and 50mg solid NH4SCN. Shake. Blue colouration in the alcohol layer Co2 is confirmed. 2. Add drops of 1 alcoholic alpha-nitroso-betanaphthol. Reddish brownprecipitate. Co2 is confirmed. 3. Add 1 drop of NH4Cl solution. Make it faintly alkaline with NH3 solution. Add 3-5 drops of dimethyl glyoxime reagent. Redprecipitate. Ni2 is confirmed. Residue-4.2 May contain Mn 2. Dissolve in 1ml dil.HNO3. Divide into 2 parts. 1. Add 2 drops of 3 H2O2. Warm to decompose excess H2O2. Cool. Add 50 mg NaBiO3. Shake. Allow to stand. The solution turns purple Mn 2is confirmed. 2. Take the other part in a boiling tube with 0.5 ml H2O. Add 2 drops of 3 H2O2. Boil to decompose excess H2O2. Cool. Add 0.5ml con.HNO3 and 250mg PbO2. Boil. Allow to stand A purple solution is formed. Mn2 is confirmed. Centrifugate-4.2 May contain Zn2. Divide into 3 parts. 1. Pass H2S. White precipitate. (ZnS). Zn2 is confirmed. 2. Just acidify with dil. H2SO4, add 5 drops of 0.1 CuSO4 solution and 5 drops of ammonium mercury thiocyanate reagent. Stir. Violet precipitate. Zn2 is confirmed. 3. Just acidify with dil.H2SO4. Add a drop of dil. cobalt nitrate solution, 0.5ml of ammonium mercury-thiocyanate reagent. Stir. Pale blue precipitate. Zn2 is confirmed.
18
ANALYSIS OF GROUP V The group V precipitate.
(Residue-5) may contain Ba2, Ca2 and Sr 2.
Dissolve the precipitate in 1 ml of dil. acetic
acid . Warm. Divide into three parts and test as
follows.
Part 1 Add a few drops of K2Cr2O7 ? Yellow ppt of BaCrO4. Ba2 is confirmed. Dissolve the residue in a few drops of conc. HCl, Apply flame test. Yellowish green flame. Ba2 is confirmed. Part 2 To 1ml of the solution add1ml of saturated ammonium sulphate solution followed by 0.1 g sodium thiosulphate. Heat on water bath for 5 minutes. White ppt. of SrSO4, Sr2 is present. Add a few drops of conc. HCl to the ppt, apply flame test. Crimson flame. Sr2 is confirmed. Part 3 Divide into two parts - 1. Add NH3 solution to get the smell of ammonia. Add ammonium oxalate solution. Warm. White precipitate. Ca2 is confirmed. 2. The other portion is evaporated to a pasty mass. Add 1 drop of conc. HCl. Apply flame test. Brick-red flame. Ca2 is confirmed.
19
ANALYSIS OF GROUP VI The centrifugate from Group
VI is evaporated to dryness. A white residue is
obtained. Group VI cations are present.
Dissolve in a few drops of dil. HCl. Add 1ml of
water. Divide into 3 parts.
1. Add 3 drops NH4Cl, and NH4OH till alkaline. Add 4 drops of Na2HPO4. White precipitate. Mg2 confirmed. 2. Add 2 drops of Magneson reagent and NaOH solution drops till alkaline. A blue precipitate. Mg2 is confirmed. 3. Add a few drops of 2 oxine solution ( 8 hydroxyquinoline). Warm. Pale yellow precipitate of Mg oxinate. Mg 2 Present.