Barometer PowerPoint PPT Presentation
Title: Barometer
1
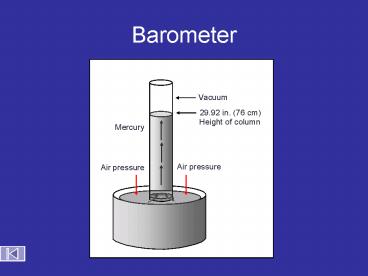
Barometer
Vacuum
29.92 in. (76 cm)
Height of column
Mercury
Air pressure
Air pressure
2
Barometer
Vacuum
29.92 in. (76 cm)
Height of column
Air pressure
Air pressure
Mercury
3
How to Measure Pressure
- Barometer
- measures atmospheric pressure
vacuum
PHg
Patm
Courtesy Christy Johannesson www.nisd.net/communic
ationsarts/pages/chem
4
air pressure
barometer device to measure air pressure
5
Barometer
Zumdahl, Zumdahl, DeCoste, World of Chemistry
2002, page 401
6
Barometer
Water column (34.0 ft. high or 10.4 m)
- Mercury filled
- 760 mm 1 atm
- Water filled
- 10400 mm 1 atm
Atmospheric pressure
Mercury column (30.0 in. high or 76 cm)
The barometer measures air pressure
7
Barometers
Mount Everest
Sea level On top of Mount Everest
Sea level
8
Boiling vs. Evaporation
Boiling point atmospheric pressure vapor
pressure
AIR PRESSURE 15psi
Revolutionary process - fast
Lyophilization freeze drying
VAPOR PRESSURE 15 psi
Evaporation molecules go from liquid to gas
phase
Evolutionary process - slow
gas
liquid
9
Boiling Point on Mt. Everest
Water exerts a vapor pressure of 101.3 kPa at a
temperature of 100 oC. This is defined as its
normal boiling point vapor pressure
atmospheric pressure
x kPa 253 mm Hg (101.3 kPa) 33.7 kPa
(760 mm Hg)
10
Boiling Point on Mt. Everest
Water exerts a vapor pressure of 101.3 kPa at a
temperature of 100 oC. This is defined as its
normal boiling point vapor pressure
atmospheric pressure
61.3oC
78.4oC
100oC
101.3
93.3
80.0
66.6
chloroform
ethyl alcohol
53.3
Pressure (KPa)
40.0
water
26.7
13.3
0
10
20
30
40
50
60
70
80
90
100
Temperature (oC)
101.3 kPa
x kPa 253 mm Hg
33.7 kPa
760 mm Hg
11
Boiling Point on Mt. Everest
Water exerts a vapor pressure of 101.3 kPa at a
temperature of 100 oC. This is defined as its
normal boiling point vapor pressure
atmospheric pressure
61.3oC
78.4oC
100oC
101.3
93.3
80.0
66.6
chloroform
ethyl alcohol
Pressure (KPa)
53.3
40.0
water
26.7
13.3
0
10
20
30
40
50
60
70
80
90
100
Temperature (oC)
101.3 kPa
x kPa 253 mm Hg
33.7 kPa
760 mm Hg
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.