PC4259 Chapter 4 Adsorption on Solid Surfaces PowerPoint PPT Presentation
Title: PC4259 Chapter 4 Adsorption on Solid Surfaces
1
PC4259 Chapter 4Adsorption on Solid Surfaces
Catalysis
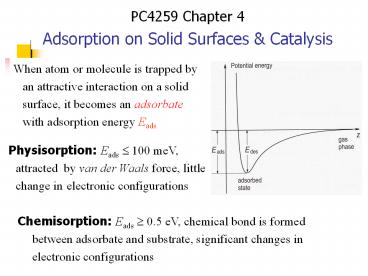
When atom or molecule is trapped by an attractive
interaction on a solid surface, it becomes an
adsorbate with adsorption energy Eads
Physisorption Eads ? 100 meV, attracted by van
der Waals force, little change in electronic
configurations
Chemisorption Eads ? 0.5 eV, chemical bond is
formed between adsorbate and substrate,
significant changes in electronic configurations
2
Van der Waals (London) Interaction
Neutral atoms can induce (fluctuating) dipole
moments in each other
p1
p2
r
(? polarizability)
?p1? 0 but ?p12? ? 0
Interaction between mutual induced dipoles
Full potential energy
Repulsion between atoms at small distance
Lennard-Jones potential
3
Physisorption Potential
Modeled as the interaction of an induced
adsorbate dipole with its image dipole
Physisorption potentials of He atoms on some
metals calculated with jellium model
4
Chemisorption electronic structures of adsorbate
surface go through significant reconfiguration,
form chemical bond (metallic, covalent or ionic)
DFT calculation results of charge densities of
some chemisorbed atoms on a jellium substrate
E-donation from Li E-capture by Cl
In chemisorption, Eads 1 eV/atom 96.5 kJ/mol
23.1 kcal/mol
5
Dissociative chemisorption a molecule
dissociates, and the breaking species form
chemical bonds with surface (e.g., O2 ? O O on
Fe)
Dissociation energy of molecule AB
Ediss
Ediss 4.5 eV, 5.2 eV and 9.8 eV for H2, O2 and
N2
Dissociative adsorption energy
For O2 on Fe, since O Fe bond strength is 4.2
eV, the dissociative Eads is 3.2 eV
6
Transition Between Physisorption Chemisorption
states
Z
Molecular physisorption dissociative
chemisorption potential curves intersect at
transition point z
Activation energy for chemisorption Eact
Precursor state for chemisorption
Barrier from precursor to chemisorption state
?a Eact ?d
7
Evolution of molecular bond in chemisorption
bridge site ?a 0.5 eV
Transition point
on-top site ?a 0.7 eV
H2 on Pd(100), bridge site on-top site
H2 on Cu(100)
8
Desorption from Surface
- Desorption Adsorbed species gain sufficient
energy to leave the surface - Thermal desorption desorption process activated
by thermal energy (e.g., by raising temperature) - Stimulated desorption desorption activated by
energy transfer from photons, electrons, ions, - Reaction before desorption adsorbed atoms form
molecules, then the molecules leave surface
9
Activation Energy for Desorption
Physisorbed non-dissociative chemisorbed
species Edes Eads
Desorption of recombined dissociative chemisorbed
species Edes Eads Eact
10
Arrangement of Adsorbates on Surface
Depends on coverage ?, adsorbate-substrate
adsorbate-adsorbate interactions, and T
? , in unit of ML (monolayer), can be measured
using XPS, AES or EELS
Low ? high T, ? 2-D gas phase
High ? low T, ? 2-D order phase
High ? high T, ? 2-D liquid phase
Phase diagram transition
11
Types of Adsorbate-adsorbate Interactions
- Van der Waals attraction between mutually
induced dipoles, important only for physisorbed
inert gas at low T - Dipole force between permanent dipoles of
adsorbed molecules (e.g. H2O, CO, NH3), or due to
charge transfer in bond formation, often
repulsive due to parallel dipoles - Orbital overlap between adsorbates at
neighboring sites, often repulsive due to Pauli
exclusion - Substrate-mediated interactions Adsorbate
disturbs electronic or mechanic structures (e.g.
charge transfer or elastic distortion) at nearby
sites, make them more favorable or unfavorable
for others to occupy, corresponding effective
attraction or repulsion - Mainly consider nearest neighbor (nn) and next
(or 2nd) nearest neighbor (nnn) interactions
12
If nn-interaction repulsive but nnn-interaction
is attractive ?
Quite Common
H2 on graphite at low T
13
Adsorption sites on hexagonal surfaces of metals
CO take on-top sites on Rh(111), but bridge sites
on Ni(111)
14
Si(111)
-Ga
Each Ga atom bonds with three Si atoms on
surface, so all Si dangling bonds are saturated,
while the dangling bond on top of a Ga atom is
completely empty, satisfying electron counting
rule
15
Si(111)
-Pb
STM image
More than one adsorbate may be accommodated in
each supercell
Need both STM (or LEED) and XPS (or AES) data
16
Si(111)
-Sb
trimer
17
Superstructures formed by both adsorbed
substrate atoms
Simple two-layer case
fl fu 1
fu
fl
Si(111)
-Ag
18
Dynamic Adsorption Desorption Measurements
To find out binding energy, activation barrier
for adsorption, etc.
A flux F can come from a gas-phase ambient of
pressure p
A flux can also be generated by a gas doser, a
molecule beam or an evaporator in vacuum
At constant F or p for a period t, Ft or pt is
the total exposure
Unit of Ft monolayer (ML)
pt is often in unit of Langmuir (L), 1 L 10-6
torr-s
19
Adsorption Kinetics
Under a flux F, surface coverage ? increases at a
rate
Probability of sticking or sticking coefficient
- ? condensation coefficient, reflecting effects
of orientation (steric factor), energy
dissipation of adsorbed particles - f(?) coverage factor, represents the
probability of finding available adsorption
sites. Sticking may be hindered by adsorbates
already on surface - exp(-Eact/kT) Boltzmann factor, comes in if
there is a barrier for adsorption
20
Langmuir adsorption model each adsorption site
only accommodate 1 particle, ? ? 1 ML
Non-dissociative adsorption (n 1)
?
Dissociative adsorption of diatomic molecule (n
2)
Dissociative adsorption of n-atom molecules
n order of adsorption
(non-activated)
21
In physisorption or atomic chemisorption with
Edes gtgt kT, initial sticking coefficient s0 ? 1
independent of T
In dissociative chemisorption with a
physisorption precursor state of binding energy
?d and a barrier to chemisorption ?a, s0 depends
on T
Molecule precursors of coverage ?p
Rate to desorb
Rate to chemisorption
Initial sticking coefficient
22
Initial sticking coefficient in dissociative
chemisorption
Eact ?a - ?d from Arrhenius plot ln(1/s0 -1)
vs 1/T
23
Coverage factor in nth-order activated
chemisorption
If precursor physisorption can occur at all
sites, conversion to chemisorption requires n
empty sites, introducing ka(1 - ?)n factor
Overall coverage factor
(K ka/kd)
24
Sb4 chemisorption on Si surfaces (n 4)
T-dependence of K
Case of decreasing K at higher T, indicating ea gt
ed,
25
Mass Spectrometer for desorption measurement
Mass spectrometer
Sample
TemperatureControl
Isothermal desorption T fixed
Programmed desorption T varies with time
26
Desorption rate
If adsorbates occupy identical sites, for
nth-order desorption (e.g. n adsorbed atoms
recombine first and then desorb as a molecule)
(Polanyi-Wigner equation)
n 0 desorption of 2-D dilute gas in
equilibrium with a 2-D solid, e.g. adatoms on a
multilayer film
In isothermal desorption (T fixed)
27
Isothermal desorption of 2-D gas of Ag in
equilibrium with 3 different 2-D solid phases
Edes from Arrhenius plot
28
1st-order (n 1) Isothermal Desorption
attempt frequency 1013 s-1
(?0 1 ML, Eads 3 eV)
2nd-order (n 2, e.g. O O ? O2?) kinetics for
associative diatomic molecular desorption
(in Homework 8)
29
Temperature Programmed Desorption (TPD)
Analyze bonding and reaction properties of
adsorbed species
Linear T ramping T(t) T0 ?t
- When T is low, desorption rate is low due to
Boltzmann factor - At a very large t (or T), surface is run out of
adsorbates, desorption rate is also low. - At Tm, desorption flux reaches a peak
30
0th-order TPD
TPD n 0
Peak is reached right be before all adsorbates
have desorbed
First-order TPD
TPD n 1
Peak at
In 1st-order TPD, Tm is independent of ?0
31
Edes from 1st-order TPD
TPD n 1
1013 s-1
2nd-order TPD
?m
TPD n 2
Tm decreases as ?0 increases
Spectra are more symmetric
32
TPD spectra show a combination of a few kinetic
models
Multilayer desorption 0th-order followed by
1st-order
Inhomogeneous substrate
33
Adsorption Isotherm
The coverage ? on a surface in equilibrium with a
gas ambient of pressure p satisfies
, or
with
In first-order Langmuir adsorption system
Langmuir adsorption isotherm
34
HREELS for adsorbate bond configurations of
atoms and molecules
Also can be detected with optical scattering
method Bond orientation from polarization
dependence
Large shift
35
Electron Stimulated Desorption (ESD)
Through excitation of electronic system of
adsorbates
Desorption of ionic or neutral species
36
Electron Stimulated Desorption Ion Angular
Distribution (ESDIAD)
Flying away direction
At low ?
0.5lt?lt1
e
H
H
O
0.2 lt ? lt1
H ESDIAD from Ru(0001)
37
Adsorption Induced Work Function Variation
Dipole moment p qd intrinsic induced
In-plane dipole has no effect
38
Cs-Induced Work Function Variation
Cs large ion size, e-donor
Dipole-dipole interaction introduces a
depolarization factor
? polarizability
39
Cs adsorption on Semiconductor
With
On p-type GaAs
Bands bend downward
Evac ? EC
negative electron affinity
high-flux photo-cathode
40
Adsorption Induced Change in LDOS near EF
Ni(111)-O
Depletion of LDOS at EF
0
6 L
100 L
1000 L
Surfactants adsorbates to purposely modify
surface property
41
Kinetic Barrier in Chemical Reaction
CO ½O2 ? CO2
CO oxidation
Energy gain ?Hr 283 kJ/mol
O2 dissociation barrier 5 eV
Haber-Bosch synthesis of NH3
½N2 3/2H2 ? NH3
Energy gain ?Hr 46 kJ/mol
Find a reaction path with lower barriers
N2 dissociation barrier 9.8 eV!
42
O2, H2 and N2 may easily dissociate when adsorbed
on some surfaces
Basis of Heterogeneous Catalysis Chemical
reaction via adsorption-dissociation-reaction-deso
rption path often only encounters moderate
barriers
Catalyst accelerates certain chemical reaction,
but is not consumed in reaction
43
Gerhard Ertl 2007 Nobel Prize in Chemistry
for his pioneering studies of chemical processes
on solid surfaces. He developed quantitative
description of how H organizes on surfaces of
catalytic metals such as Pt, Pd, and Ni. He also
produced key insights into mechanism of
Haber-Bosch process of NH3 synthesis
Haber-Bosch synthesis of NH3 on Fe
N2 dissociation not a major obstacle, but H
addition to N is up-hill
44
CO oxidation on Pt(111) main barrier now is
only 105 kJ/mol, while in gas phase O2
dissociation requires 490 kJ/mol
Catalyst to convert CO to CO2, NO to N2 and HC to
H2O in a car exhaust contains Pt, Pd, Rh and Ir
45
LDOS(EF), d-band center Reactivity
LDOS at EF and surface reactivity are closely
correlated
E
Noble metal EF
Transition metal EF
d-band
sp-band
Downward shift of d-band center increase of N2
dissociation barrier on Ru(0001) induced by
adsorption of N, O or H,
DOS at EF in noble or transition metals
46
K as electronic promoter in NH3 synthesis
Enhance LDOS at EF
Lower physisorption potential curve of N2
Raise nitrogen sticking probability by ?102
47
Poisoning of catalyst
Poisoning often occurs due to coverage of S or
graphitic C
On clean Pd(100), H2 dissociation is barrier-less
On p(2?2)S/Pd(100), H2 dissociation barrier 0.1
eV
On c(2?2)S/Pd(100), H2 dissociation barrier 2
eV, blocked
S adsorption shifts Pd d-band downward, surface
becomes more repulsive for H2 adsorption
dissociation
48
General suitability of material as catalyst
should be just moderately reactive
Methanation of CO
CO 3H2 ? CH4 H2O
Fischer-Tropsch reaction facilitated by Fe-Co
catalysts doped with K Cu
Volcano curve