Biochemistry by Mary Campbell - PowerPoint PPT Presentation
1 / 51
Title: Biochemistry by Mary Campbell
1
Chapter 3
2
Classification of Matter
3
Classification of Matter
- Element a substance that consists of atoms with
the same number of protons in the nucleus - there are 114 known elements
- of these, 88 occur in nature the others have
been made by chemists and physicists - their symbols consist of one or two letters
4
The Elements
- Monatomic elements consist of single atoms
- Diatomic elements there are seven elements that
occur as diatomic molecules - Polyatomic elements some elements have three or
more elements per molecule
5
Classification of Matter
- Compound a pure substance made up of two or more
elements in a fixed mass ratio - Formula of a compound tells us the counting
number ratios of its constituent elements and
identifies each element by its atomic symbol. - NaCl the ratio of sodium atoms to chlorine atoms
in sodium chloride is 11 - H2O the ratio of hydrogen atoms to oxygen atoms
in water is 21
6
Periodic Table
- Dmitri Mendeleev (1834-1907)
- arranged the known elements in order of
increasing atomic weight - he observed certain sets of properties recur
periodically - he then arranged elements with recurring sets of
properties in the same column called groups or
families - Rows are called periods
7
Periodic Table
- Fluorine, chlorine, bromine, and iodine fall in
the same column
8
Classification of Elements
9
Classification of Elements
- Metals
- are solids (except for Hg), shiny, conductors of
electricity, ductile, and malleable - form alloys
- tend to give up electrons
- Nonmetals
- except for hydrogen (H), lie on the right side of
the Periodic Table - except for graphite, do not conduct electricity
- tend to accept electrons
10
Classification of Elements
- Metalloids
- six elements are classified as metalloids boron,
silicon, germanium, arsenic, antimony, and
tellurium - they have some of the properties of metals and
some of nonmetals
11
Examples of Periodicity
- The halogens, Group 7A elements
12
Examples of Periodicity
- The alkali metals, Group 1A elements
13
Examples of Periodicity
- The noble gases, Group 8A elements
14
Laws of chemistry
- Law of conservation of mass There is no
detectable change in mass in an ordinary chemical
reaction (Lavoisier, 1798) - Law of constant composition A specific chemical
compound always contains the same elements in the
same proportions by mass - Law of multiple proportions When two elements
combine to form two or more compounds, the masses
of one combining with a fixed mass of the other
are in ratios of small whole numbers
15
Daltons Atomic Theory
- All matter is composed of very tiny particles,
which Dalton called atoms - Atoms of different elements are different
- Compounds are atoms combined in whole number
ratios - Ionic compounds
- Molecular compounds
- Chemical reactions only rearrange atoms in how
they are combined.
16
Evidence for Daltons Theory
- Law of Conservation of Mass
- Law - mass can be neither created nor destroyed
- Theory - if matter is made up of indestructible
atoms, then any chemical reaction just changes
the attachments among atoms, but does not destroy
nor change the atoms identities
17
Atoms exist Now what?
- If atoms exist, how can we characterize them?
- Study the particles they emit
- R. A. Millikan, an American physicist, studied
electrons separate from the atom as they became
attached to oil droplets falling towards a
magnet. He was able to calculate the charge of
one electron to 5 sig figs as 1.6022 x 10-19 C - Link to Video
18
Atoms exist Now what?
- If atoms exist, how can we characterize them?
- Study the particles they emit
- J. J. Thomson bended the path of a stream of
electrons in a cathode ray tube enabling the
direct calculation of an electrons mass.
19
Atoms exist Now what?
- If atoms exist, how can we characterize them?
- Study the particles they emit
- Ernest Rutherford -postulated the nuclear atom
after experimenting with bombarding thin foils of
metals with alpha rays. - Link to Video
20
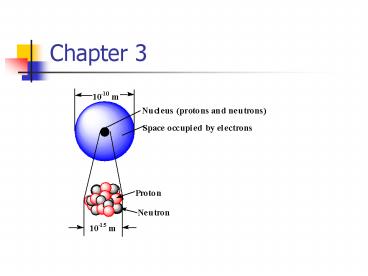
Atom internals
- Protons and neutrons are found in the nucleus,
and electrons are found as a cloud outside the
nucleus
21
- Diameter of a nucleus is only about 10-15 m.
- Diameter of an atom is only about 10-10 m.
22
Mass and Atomic Numbers
- Mass number the number of protons plus neutrons
in the nucleus of an atom - Atomic number the number of protons in the
nucleus of an atom - a carbon atom of this composition is referred to
as carbon-12
23
Isotopes
- Isotopes atoms with the same number of protons
but a different number of neutrons - carbon-12 has 6 protons and 6 neutrons
- carbon-13 has 6 protons and 7 neutrons
- carbon-14 has 6 protons and 8 neutrons
- Most elements found on Earth are mixtures of
isotopes - chlorine is 75.77 chlorine-35 and 24.23
chlorine-37
24
Atomic Weight
- Atomic weight the weighted average of the masses
in amu of the isotopes of an element found in
nature - example chlorine is 75.77 chlorine-35 and
24.23 chlorine-37
25
Classification of Elements
- Metals
- are solids (except for Hg), shiny, conductors of
electricity, ductile, and malleable - form alloys
- tend to give up electrons
- Nonmetals
- except for hydrogen (H), lie on the right side of
the Periodic Table - except for graphite, do not conduct electricity
- tend to accept electrons
26
Classification of Elements
- Metalloids
- six elements are classified as metalloids boron,
silicon, germanium, arsenic, antimony, and
tellurium - they have some of the properties of metals and
some of nonmetals
27
Examples of Periodicity
- The halogens, Group 7A elements
28
Examples of Periodicity
- The alkali metals, Group 1A elements
29
Examples of Periodicity
- The noble gases, Group 8A elements
30
Do Electrons orbit the Nucleus?
- Do you Know that?
- Do you think that?
- Do you believe that?
31
Electrons are the key to chemistry
- Early experiments where elements were studied
lead to an understanding that each element
emitted a specific light pattern when excited.
32
Fingerprint Meaning Revealed
- The energy of electrons in an atom is quantized
- An electron in an atom can have only certain
allowed energies. - The fingerprint we see is an electron relaxing
from an excited state to a lower excited state or
the ground state thus giving off a specific
amount of energy.
33
Fingerprint Meaning Revealed
- Neils Bohr - Interpreted fingerprint as
transitions between allowed distances of an orbit
from the nucleus. That si to say electrons orbit
the nucleus! - Math works for 1 electron systems only
34
Fingerprint Meaning Revealed
- Schrodinger Interpreted fingerprint as
transitions between allowed energies of waves. - Math works for all atoms spectra plus can be
applied to molecules as well - Electron energy descriptions are called
electronic configurations. These come from the
math of the Schrodinger Equation.
35
The Wave Mechanical Model
Electrons have a dual wave/particle
nature Electrons in atoms are treated by the
wave mechanical model as standing waves around
the atom nucleus Wave mechanical calculations
describe the allowed energies for electrons in
atoms as orbitals (not orbits) A single orbital
may contain a maximum of two electrons Four
quantum numbers detail electron energies in the
wave equation (but we wont do the math) No two
electrons in an atom have the same set of quantum
numbers
36
The Principal Quantum Number, n
Size of orbitals increases with increasing
n Magnitude of the average energy of electrons
contained in an orbital increases with increasing
n Permitted values of n are 1, 2, 3,,7 for the
known elements
37
The Azimuthal Quantum Number, l
l designates the orbtial type (also called a
subshell but I am no a fan) For any shell with
principal quantum number, n, the possible values
of l are 0, 1, 2, 3,,(n-1) l designates
different shapes (energies) of orbitals Sublevel
s can be designated by s, p, d, and f
corresponding to l values of 0, 1, 2, and 3
38
The magnetic quantum number, ml
ml is also called the orientational quantum
number Distinguishes orbitals within a subshell
from each other Designates orientations of
orbitals in space relative to each other For a
given value of l, possible values of ml are l,
(l-1)0-(l-1), -l
39
Spin Quantum Number, ms
Associated with a magnetic field generated by
spinning electron ms may be either 1/2 or
-1/2 Two electrons can occupy the same orbital
only if they have opposite spins so that their
magnetic moments cancel each other
40
Quantum Numbers Summarized, orbital diagrams
41
Quantum Numbers Summarized, orbital diagrams
42
Electron Configuration
- Electron configuration the arrangement of
electronic energies in the extranuclear space - Ground state the electron configuration of
lowest energy - Excited state all electronic configurations
other than the ground state - The fingerprint we see is an electron relaxing
from an excited state to a lower excited state or
the ground state thus giving off a specific
amount of energy.
43
Electron Configuration
- Electron configurations are governed by three
rules - Rule 1 orbitals fill in the order of increasing
energy from lowest to highest - elements in the first, second, and third periods
fill in the order 1s, 2s, 2p, 3s, and 3p
44
(No Transcript)
45
3.17. ELECTRON CONFIGURATIONS AND THE PERIODIC
TABLE Figure 3.21. Periodic Table Showing the
Filling of Atomic Orbitals
46
Electron Configuration
- Rule 2 each orbital can hold up to two electrons
with spins paired - with four electrons, the 1s and 2s orbitals are
filled and are written 1s2 2s2 - with an additional six electrons, the three 2p
orbitals are filled and are written either 2px2
2py2 2pz2, or they may be written 2p6
47
Electron Configuration
- Spin pairing means that electrons spin in
opposite directions
48
Electron Configuration
- Rule 3 when there is a set of orbitals of equal
energy, one orbital becomes half filled before
any of them becomes completely filled - example after the 1s and 2s orbitals are filled,
a 5th electron is put into the 2px, a 6th into
the 2py, and a 7th into the 2pz. Only after each
2p orbital has one electron is a second added to
any 2p orbital.
49
Electron Configuration
- Orbital box diagrams
- a box represents an orbital
- an arrow represents an electron
- a pair of arrows with heads in opposite
directions represents a pair of electrons with
paired spins - Example carbon (atomic number 6)
50
Electron Configuration
- Noble gas notation
- the symbol of the noble gas immediately preceding
the particular atom indicates the electron
configuration of all filled shells - Example carbon (atomic number 6)
51
Electron Configuration
- Valence shell the outermost incomplete shell
- Valence electron an electron in the valence
shell - Lewis dot structure
- dots represent valence electrons