Br2 PowerPoint PPT Presentations
All Time
Recommended
Introducing the Peplink BR2 Micro!
| PowerPoint PPT presentation | free to download
Introducing the Peplink MAX BR2 – rugged, dual-cellular router for the most reliable remote and mobile applications. As a certified platinum partner of Peplink, we bring you built-in eSIM for zero-truck-roll provisioning, dual-cellular modems, and SpeedFusion technology for unbreakable connectivity. Get yours from a certified platinum partner and distributor of Peplink, NGT Technology. Get the best of Peplink routers and Antennas from NGT tech. Visit our Amazon page : https://bit.ly/3Wlwqs5
| PowerPoint PPT presentation | free to download
Title: Alkanes Author: GWH28-DGCMP-P6RC4-6J4MT-3HFDY Last modified by: Authorized User Created Date: 1/25/2006 2:37:29 PM Document presentation format
| PowerPoint PPT presentation | free to download
Caterpillar Cat 3512B Industrial Engine (Prefix BR2) Service Repair Manual Instant Download (BR200001 and up)
Caterpillar Cat 3512B Industrial Engine (Prefix BR2) Service Repair Manual Instant Download (BR200001 and up)
Caterpillar Cat 3512B Industrial Engine (Prefix BR2) Service Repair Manual Instant Download (BR200001 and up)
0.266 moles Br2 / 1 = 0.266 limiting. 42.5 grams Br2 1 mol Br2 2 mol KBr 119 g KBr ... 1 160 g Br2 1 mol Br2 1 mol KBr = 63.2 g KBr. When the reaction is done...
| PowerPoint PPT presentation | free to view
Intermolecular Forces and Liquids and Solids Chapter 12 12.7 The compounds Br2 and ICl have the same number of electrons, yet Br2 melts at -7.2oC, whereas ICl melts ...
| PowerPoint PPT presentation | free to download
Below a threshold energy, Si etching rate is independent of E (PAE regime). PAE rate of Si varies by gas mixture: Cl2/HBr Cl2, Cl2/Br2 HBr/Ar Br2/Ar Double headed ...
| PowerPoint PPT presentation | free to download
Preparation of Organic Halides Halogenation or Hydrohalogenation of an Alkene, Alkyne, or Benzene Ring e.g. CH2 = CH2 + Br2 CH2CH2 | | Br Br
| PowerPoint PPT presentation | free to view
Highly unsaturated (r db = 4), yet Br2 or HBr does not add across multiple ... Reacts with Br2 in presence of FeBr3 catalyst by ... pyridine pyrrole imidazole ...
| PowerPoint PPT presentation | free to view
conversion of alkenes to vicinal. halohydrins, followed by treatment. with base (Section 16.10) ... Epoxidation via Vicinal Halohydrins. Br2. H2O. OH. NaOH ...
| PowerPoint PPT presentation | free to download
Cyclooctatetraene Cyclooctatetraene has four double bonds, reacting with Br2, KMnO4, and HCl as if it were four alkenes. Does NOT have delocalized double bonds.
| PowerPoint PPT presentation | free to download
Unbreakable Connectivity for Security Systems with Peplink Tired of connection problems, missing footage, and access control failures? Upgrade to Peplink’s BR2 Pro 5G router. Get fast and reliable connections for your security systems with dual 5G and smart failover. Find out how Peplink can improve your security. Get yours from a certified platinum partner and distributor of Peplink, NGT Technology. For inquiries
| PowerPoint PPT presentation | free to download
Chemical reactions are represented by chemical equations. ... Check for Diatomic Molecules - H2 - N2 - O2 - F2 - Cl2 - Br2 - I2 ...
| PowerPoint PPT presentation | free to view
F2 Cl2 Br2 I2. Se emplean Cl2 y Br2. F2 es demasiado reactivo; reacci n explosiva, dif cil ... Para ello tiene que romperse alg n enlace, de esta manera: ...
| PowerPoint PPT presentation | free to view
Formulas. BY: JIMMY T. Types. Diatomic H2, N2, O2, F2, Cl2, Br2, I2. Polyatomic ions ... Diatomic, Polyatomic, Molecular, and. Empirical. ...
| PowerPoint PPT presentation | free to view
Which of the following compounds contains a polar covalent bond -NaF -HCl -Br2 -MgO Which of the following compounds contains an ionic bond -NH3 -H2O -CaO -CH4 ...
| PowerPoint PPT presentation | free to view
IN aliphatic acid. Hell-Volhard-Zeliniki reaction. It occurs in aliphatic acid at a-position. Other example. ???? ?? ??? ??? ??????? ?? Br2 ...
| PowerPoint PPT presentation | free to view
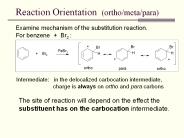
Reaction Orientation (ortho/meta/para) Examine mechanism of the substitution reaction. For benzene + Br2 : Intermediate: in the delocalized carbocation intermediate,
| PowerPoint PPT presentation | free to download
2. conversion of alkenes to vicinal. halohydrins, followed by treatment. with base (Section 16.10) ... Epoxidation via Vicinal Halohydrins. Br2. H2O. OH ...
| PowerPoint PPT presentation | free to view
Diatomic Molecules. Molecules made up of two atoms. There are 7 diatomic molecules. H2, N2, O2, F2, Cl2, Br2, I2. Hydrogen. H H H:H ...
| PowerPoint PPT presentation | free to view
... radicals formed from the reaction of hydroxyl radicals and the scavenger S at concentration [S] ... for hydroxyl radicals. Br Br. Br2 ...
| PowerPoint PPT presentation | free to view
nonmetal replaces nonmetal (-) A BC B AC. D. Single Replacement ... nonmetal nonmetal (-) free element must be more active (check activity series) Br2(l) NaCl(aq) ...
| PowerPoint PPT presentation | free to view
Chapter 24. Amines and Heterocycles Based on McMurry s Organic Chemistry, 7th edition * Hofmann Rearrangement RCONH2 reacts with Br2 and base Gives high yields of ...
| PowerPoint PPT presentation | free to view
... for bonding H2 N2 O2 F2 I2 Cl2 Br2 Polyatomic Ions A group of atoms that behave as one atom Keep together as a unit ... 1 0 +2 ... from Greek / Latin ...
| PowerPoint PPT presentation | free to view
Claisen and Cope rearrangements. Cope rearrangements and related: Beckman rearrangement ... RCONH2 reacts with Br2 and base. Gives high yields of arylamines and ...
| PowerPoint PPT presentation | free to view
Diatomic molecules are composed of exactly two atoms. Diatomic elements -elements found as diatomic molecules: H2, O2, N2, F2, Cl2, Br2 and I2 ...
| PowerPoint PPT presentation | free to view
Friedel-Crafts. Halogenation (Cl2, Br2) FeBr3 is the actual catalyst. ... Acylation. Friedel-Crafts Alkylation. Acylating Reagents: F-C Acylation. F-C Alkylation ...
| PowerPoint PPT presentation | free to view
Chemists require a unit for counting which can express large numbers of atoms ... In dealing with diatomic elements (H2, O2, N2, F2, Cl2, Br2, and I2) ...
| PowerPoint PPT presentation | free to view
... X2 to form vicinal dihalides. alkenes react with X2 in water to give vicinal ... Vicinal bromohydrins are prepared by treatment of alkenes with Br2 in water. ...
| PowerPoint PPT presentation | free to view
Requires a stronger electrophile than Br2. Use a strong Lewis acid catalyst, FeBr3. ... Electrophilic substitution reactions for nitrobenzene are 100,000 times ...
| PowerPoint PPT presentation | free to view
Diatomic molecules: H2, N2, O2, F2, Cl2, Br2 , I2 ... Check 4 to see if any of the elements are diatomic (come in pairs) ...
| PowerPoint PPT presentation | free to view
Balancing Equations H2 + Cl2 HCl Balancing Equations KI + Br2 KBr + I2 Balancing Equations CH4 + O2 CO2 + H2O Balancing Equations V2O5 + Ca V + CaO ...
| PowerPoint PPT presentation | free to download
Diatomic Elements. H2, N2, O2, F2, Cl2, Br2, and I2. Also P4 and S8. Polar Covalent ?E = 0.5 1.6 ... Diatomic elements. 100% shared. 100% covalent. Different ...
| PowerPoint PPT presentation | free to view
Diatomic 7 H2 Hydrogen gas Cl2 Chlorine gas Br2 Bromine gas O2 Oxygen gas I2 Iodine gas N2 Nitrogen gas F2 Flourine gas Diatomic 7 H2 Hydrogen gas Cl2 Chlorine gas ...
| PowerPoint PPT presentation | free to download
... (H2) atomic radius = 37 pm. Fluorine (F2) atomic radius = 64 pm ... fluorine pale yellow gas F2. chlorine pale green gas Cl2. bromine reddish-brown liquid Br2 ...
| PowerPoint PPT presentation | free to view
Leon County Schools Other titles: Arial Default Design Hexane layers on top, aqueous on bottom. What is in each vial? Cl2 , Cl2, Br2, I2 Cl2 in hexane layer on top ...
| PowerPoint PPT presentation | free to view
How to solve this problem? Make Br2 more electrophilic. Aromatic. Not aromatic. FeBr3 ... Other Electrophiles: Friedel-Crafts Acylation. Write out the complete ...
| PowerPoint PPT presentation | free to view
Atoms bond with each other to become more. chemically stable than they were before they ... 4. There are 7 Diatomic elements (N2, O2, F2, Cl2, Br2, I2, H2) ...
| PowerPoint PPT presentation | free to view
Prepare the alkene (large variety of preparation methods ... Destroy the double bond by adding Br2 thus generating a ... similar to pentane) 17. Synthesis ...
| PowerPoint PPT presentation | free to view
When elements combine to form a new substance with properties different from ... There are 7 diatomic elements. H2, N2, O2, F2, Cl2, Br2, and I2. Ionic Compounds ...
| PowerPoint PPT presentation | free to view
... cell = E ox E red. Electrochemistry: ... 2Br-(aq) Br2(l) 2e- E ox = -1.06. F2(g) 2e- 2F-(aq) E red = 2.87 ... 4Fe2 (aq) 4Fe3 (aq) 4e- E ox = -0.77 V ...
| PowerPoint PPT presentation | free to view
Electrophilic addition reactions of alkenes. There is some narration so turn the sound on! ... The d end of the Br2 acts as an electrophile. Q2. ...
| PowerPoint PPT presentation | free to view
Phase symbols (s, l, g, aq) are used to ... Polyatomic Elements: P4 (s) and S8 (s) Diatomic Elements: H2 (g), N2 (g) O2 (g), F2 (g), Cl2 (g), Br2 (l), I2(s) ...
| PowerPoint PPT presentation | free to view
X must be a halogen. CH4 Cl2 CH3Cl HCl. methane. chloromethane. 20.6 Reactions of Alkanes ... Halogenation: addition of halogen atoms. CH2=CHCH3 Br2 CH2BrCHBrCH3 ...
| PowerPoint PPT presentation | free to view
Pollution is the action of environmental contamination with man ... Bromine is regenerated. 2 HBr Br2 H2. H2S H2 1/2 S2. Electrochemical gas purification ...
| PowerPoint PPT presentation | free to view
Mg(s) FeSO4(aq) Cl2(g) KBr(aq) Fe(s) MgSO4 (aq) Br2 (aq) KCl(aq) MgSO4 (aq) Fe(s) ... BaCl2(aq) KBr(aq) Fe(NO3)3(aq) Ca3(PO4)2(s) CuBr2 (aq) KCl(aq) ...
| PowerPoint PPT presentation | free to download
Hydrogen and oxygen are diatomic elements. Their subscripts cannot be changed. ... Remember the diatomic elements: H2, N2, O2, F2, Cl2, Br2, and I2. Writing and Naming ...
| PowerPoint PPT presentation | free to view
Treated Sea water then passes into a Blowing out tower: ... 2HBr(aq) Cl2(g) Br2(g) 2HCl(aq) Chlorine oxidises the Bromide in HBr to bromine. ...
| PowerPoint PPT presentation | free to view
Sharing of electrons in a molecule (non-metals) Can be ... 'like Siamese twins' - stable as a pair (they are never by themselves) - H2 O2 Br2 F2 I2 N2 Cl2 ...
| PowerPoint PPT presentation | free to view
The diatomic molecule oxygen, O2, is an example of a molecule with a double ... All of the diatomic molecules (H2, N2, O2, F2, Cl2, Br2, I2) contain nonpolar ...
| PowerPoint PPT presentation | free to view
http://www.consolidatedroofing.co.uk/ Consolidated roofing is one of the leading roofing companies Kent that provides domestic roofing services at affordable charges. There roofers are experienced and certified and hence, offer quality services. 21 Bradford close bromley kent ,BR2 8NR,United Kingdom
| PowerPoint PPT presentation | free to download
Kelly Chance, Thomas Kurosu, Trevor Beck, Andreas Richter, Michel van Roozendael, ... OMI Overpass. OMI Overpass. OMI Column (1013 mol/cm2) DC8 O3. DC8 Alt. DC8 Br2 ...
| PowerPoint PPT presentation | free to download
used to separate reactants and/or products. 'yields' ... Diatomic Molecules. H2. N2. O2. F2. Cl2. Br2. I2. 71 st Rule. Start with element 7. Forms the shape 7 ...
| PowerPoint PPT presentation | free to view
In both cases, Br2 reacts with the C = C functional group in exactly the same way. ... They are also called aliphatic compounds. Straight-chain alkanes (normal alkane) ...
| PowerPoint PPT presentation | free to view