Delocalized PowerPoint PPT Presentations
All Time
Recommended
p bonds move depending on the structure. The p electrons are delocalized ... Scissoring. Rocking. Wagging. Twisting. http://en.wikipedia.org/wiki/Infrared_spectroscopy ...
| PowerPoint PPT presentation | free to view
Diatomic molecules: The bonding in H2 ... Diatomic molecules: Homonuclear Molecules of the Second Period ... Diatomic molecules: MO diagrams for Li2 to F2 ...
| PowerPoint PPT presentation | free to download
Organic Chemistry 6th Edition Paula Yurkanis Bruice Chapter 7 Delocalized Electrons and Their Effect on Stability, Reactivity, and pKa More About Molecular
| PowerPoint PPT presentation | free to view
Delocalized electrons Sea of mobile electrons Metallic Bonds Subscripts in chemical formulas Lewis Dot Structures for Ionic Compounds Lewis Dot Structures for NaCl ...
| PowerPoint PPT presentation | free to download
Cyclooctatetraene Cyclooctatetraene has four double bonds, reacting with Br2, KMnO4, and HCl as if it were four alkenes. Does NOT have delocalized double bonds.
| PowerPoint PPT presentation | free to download
Aliphatic (fatty) Aromatic (fragrant) Open-chain cyclic compounds ... cyclic clouds of delocalized p electrons above and below the ...
| PowerPoint PPT presentation | free to view
Carbon & Hydrocarbons Chapter 22 Vocabulary Diamond Graphite Fullerenes Delocalized electrons Organic compounds Catenation Hydrocarbons Isomers Structural formula ...
| PowerPoint PPT presentation | free to view
Organic Chemistry Chapter 7 - Resonance Electron Delocalization and Resonance Localized electrons = restricted to a particular region Delocalized electrons do not ...
| PowerPoint PPT presentation | free to view
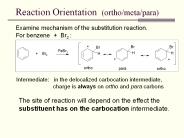
Reaction Orientation (ortho/meta/para) Examine mechanism of the substitution reaction. For benzene + Br2 : Intermediate: in the delocalized carbocation intermediate,
| PowerPoint PPT presentation | free to download
Ionic, Metallic and Covalent Bonding Writing and Naming Formulas good conductors of heat and electricity; electrons mobile delocalized electrons interact with light ...
| PowerPoint PPT presentation | free to view
primary amine. (N is a locant-it is not alphabetized, but ... Lone pair on amine nitrogen is conjugated with p-nitro group more delocalized ...
| PowerPoint PPT presentation | free to view
Delocalized electrons electrons that freely move around; empty orbitals ... Ductility. Heat of vaporization. Lewis structures. Valence electrons by dots. ...
| PowerPoint PPT presentation | free to view
The Mechanisms of Photosynthetic Light Harvesting and. Non-Photochemical Quenching ... 2-3 Bchl a. The Disorder-Exciton-Redfield model. Delocalized states ...
| PowerPoint PPT presentation | free to view
secondary, and tertiary amine is only 0.3 pK units. Effect of Structure on Basicity ... Lone pair on amine nitrogen is conjugated with p-nitro group more delocalized ...
| PowerPoint PPT presentation | free to download
Polyatomic molecules and Delocalized Bonding. Valence Bond: works well for ... Conjugation alternating double bonds. 1,3 butadiene. 4 carbons. 2 double bonds ...
| PowerPoint PPT presentation | free to download
4.4 Metallic bonding 4.4.1 Describe metallic bond as the electrostatic attraction between a lattice of positive ions surrounded by delocalized valence electrons.
| PowerPoint PPT presentation | free to view
Number of hybrid orbitals is equal to number of pure atomic orbitals used in the ... Delocalized molecular orbitals are not confined between two adjacent bonding ...
| PowerPoint PPT presentation | free to view
DFT shows good agreement with MP2, but delocalizes the charge, ... Car ... LDA shows important overbinding, while GGA is in good agreement with higher ...
| PowerPoint PPT presentation | free to view
Type of Structure. Solubility in. Water. Electrical ... e- are delocalized among metal atoms. very high. yes (any form) no. malleable, ductile, lustrous. solid ...
| PowerPoint PPT presentation | free to view
Bond that results when metal atoms release their valence electrons to ... Overlap of orbitals allows outer electrons to roam freely through metal (delocalized) ...
| PowerPoint PPT presentation | free to view
Resonance: Delocalized Electron-Pair Bonding - I Ozone : O3..... O O..... O O.. O O.... II I Resonance Hybrid Structure.. O..... O O One pair of electron s resonances
| PowerPoint PPT presentation | free to view
Benzene. Molecular Orbital Theory Delocalized MO's. Molecular Motion, ... Energy Levels, Spectroscopy, & Molecular Properties. Quantized Energy Levels. Atoms ...
| PowerPoint PPT presentation | free to view
Paul R. C. Kent and Alex Zunger. Solid State Theory Group. National Renewable Energy Laboratory ... Kent and Zunger PRL 86 2613 (2001) Localized-Delocalized ...
| PowerPoint PPT presentation | free to view
Molecular Orbital Theory Delocalized p MO's. Combine: Localized for s ; ... Conjugation alternating double bonds. 1,3 butadiene. 4 carbons. 2 double bonds ...
| PowerPoint PPT presentation | free to view
Breathing Orbital Valence Bond (BOVB) A Valence Bond Method ... 1s 1s j j j j j j j j. Electron pairs are in delocalized orbitals. The Valence Bond model ...
| PowerPoint PPT presentation | free to view
Worksheet: Chapter 12 Homework Key 1. Determine whether the bond formed between each of the elements listed below would be ionic or covalent based on the metallic ...
| PowerPoint PPT presentation | free to download
MOT : complicated bonding analyses BF3 B : 2s 2px 2py 2pz F : 2s 2px 2py 2pz BF3 ...
| PowerPoint PPT presentation | free to view
Benzene s Unusual Structure Each carbon atom is sp2 hybridized, with a p orbital perpendicular to the plane of the six-membered ring Actual structure is a hybrid of ...
| PowerPoint PPT presentation | free to download
Valence bond theory 4 bonds from C (sp3) H (1s) all of the same energy. each ... Look at CH4 and formate HCO2- H H. C. HH. CH4 Photoelectron Spectrum ...
| PowerPoint PPT presentation | free to download
... between benzylic carbon and the ring carbons that are ortho and para to it ...
| PowerPoint PPT presentation | free to download
Lesson 54 Typical properties and structure of metal. What make metal ... When a piece of metal is hammered or pulled strongly, the atoms will over ...
| PowerPoint PPT presentation | free to view
Title: Prezentace aplikace PowerPoint Author: Viktor Sember Last modified by: vs2 Created Date: 1/21/2003 4:25:34 PM Document presentation format
| PowerPoint PPT presentation | free to download
Explain why metals are ductile and malleable. ... Metal is malleable and ductile because metallic bond is non-directional and the ...
| PowerPoint PPT presentation | free to view
Chlorophyll A absorbs light that is further on the blue end and red end ... Chlorophyll is located primarily in the mesophyll cells. Located in the chloroplasts ...
| PowerPoint PPT presentation | free to view
Structures and Properties of Substances 12 12.1 Classification of Substances According to Structures 12.2 Classification of Substances According to the Nature of Bonding
| PowerPoint PPT presentation | free to view
Chapter 9 Molecular Geometries and Bonding Theories*
| PowerPoint PPT presentation | free to download
Single and Multiple Bonds in Carbon Compounds. sp3 hybridization on C ... These absorb light at longer. wavelength- sometimes even in visible (human eye's light ...
| PowerPoint PPT presentation | free to download
Title: PowerPoint Presentation - Organic Chemistry Fifth Edition Subject: Sections 24.1-24.3 Author: Francis A. Carey Last modified by: r r Created Date
| PowerPoint PPT presentation | free to view
Chapter 15 Benzene and Aromaticity Aromatic was used to described some fragrant compounds in early 19th century Not correct: later they are grouped by chemical ...
| PowerPoint PPT presentation | free to view
Phenols 24.1 Nomenclature Nomenclature named on basis of phenol as parent substituents listed in alphabetical order lowest numerical sequence: first point of ...
| PowerPoint PPT presentation | free to view
Title: Chapter 9 Molecular Geometries and Bonding Theories Author: John Bookstaver Last modified by: Software Manager Created Date: 3/7/2005 2:11:13 AM
| PowerPoint PPT presentation | free to download
29.2 Nomenclature of the Derivatives of Benzene. 29.3 Structure of Benzene and ... Benzo(a)pyrene (C20H12) New Way Chemistry for Hong Kong A-Level Book 3A. 45 ...
| PowerPoint PPT presentation | free to view
Multiple Bonds In triple bonds, as in acetylene, ... Chapter 9 Molecular Geometries and Bonding Theories Author: John Bookstaver Last modified by: LPS Created Date:
| PowerPoint PPT presentation | free to view
Chapter 9 Chemical Bonding * Mg ions and oxide ions are smaller than K ions and Cl ions. Also, Mg and O have 2+ and 2- charges, respectively. K and Cl only have ...
| PowerPoint PPT presentation | free to view
Forbidden. Zone! Energy, E. 1023 ns. Atomic Orbitals. 3 x 1023 np ... Small forbidden zone between filled band and conduction band. Filled band of. 2 x 1023 sp3 ...
| PowerPoint PPT presentation | free to download
Title: PowerPoint Presentation Last modified by: user Document presentation format: Other titles: Times New Roman Symbol CMR10 Georgia ...
| PowerPoint PPT presentation | free to download
Arenes and Aromaticity 16 16 16 21 22 22 23 22 22 22 26 27 27 28 29 30 31 31 36 31 31 31 16 16 16 16 16 16 1 3 4 5 5 5 5 2 5 6 7 7 7 8 8 8 8 8 8 8 2 2 1 15 16 16 18 ...
| PowerPoint PPT presentation | free to view
Benzene produces only one monobrominatedcompound All 6 carbon-hydrogen bonds are equivalent in benzene Specific Electrophilic Aromatic : Substitution Reactions Side ...
| PowerPoint PPT presentation | free to download
Role of Anderson localization in the QCD phase transitions Antonio M. Garc a-Garc a ag3@princeton.edu Princeton University ICTP, Trieste We investigate in what ...
| PowerPoint PPT presentation | free to download
Ultra-low Thermal Conductivity of Layered Crystals. Pawel Keblinski, ... Alumina. copper. diamond. YSZ. Phonons Phonons electrons Phonons = 1/3 c v ...
| PowerPoint PPT presentation | free to view
Molecular Geometry and Bonding Theories Brown, LeMay Ch 9 AP Chemistry Monta Vista High School Ch 9 PS #8, 10, 16, 20 (and draw overall dipole moments), 30, 34, 36 ...
| PowerPoint PPT presentation | free to download
Molecular Geometry and Bonding Theories Brown, LeMay Ch 9 AP Chemistry Monta Vista High School Ch 9 PS #8, 10, 16, 20 (and draw overall dipole moments), 30, 34, 36 ...
| PowerPoint PPT presentation | free to download
Molecular Geometry. Bonding. Covalent bonds occur when atoms are at an ideal ... one covalent bond, polarity depends on individual bonds and shapes of molecules. ...
| PowerPoint PPT presentation | free to view
Tonight Night, 5:30 pm - ?, Seaver N Auditorium. Tuesday, 12 noon -1:30, Frank ... energy of a mole of ionic solid compound compared to a mole of the diatomic ...
| PowerPoint PPT presentation | free to download
The Birch Reduction Dr. Wolf's CHM 201 & 202 Birch Reduction of Benzene Product is non-conjugated diene. Reaction stops here. There is no further reduction.
| PowerPoint PPT presentation | free to view