Ch14' The Ideal Gas Law and Kinetic Theory - PowerPoint PPT Presentation
1 / 54
Title:
Ch14' The Ideal Gas Law and Kinetic Theory
Description:
... versus-volume plot for a gas at a constant temperature is called an isotherm. For an ideal gas, each isotherm is a plot of the equation P = nRT/V = constant/V. ... – PowerPoint PPT presentation
Number of Views:160
Avg rating:3.0/5.0
Title: Ch14' The Ideal Gas Law and Kinetic Theory
1
Ch14. The Ideal Gas Law and Kinetic Theory
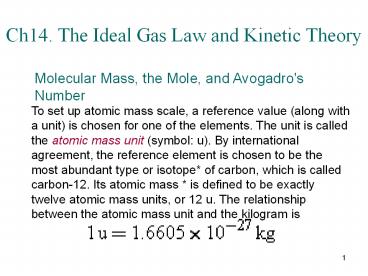
Molecular Mass, the Mole, and Avogadro's Number
To set up atomic mass scale, a reference value
(along with a unit) is chosen for one of the
elements. The unit is called the atomic mass unit
(symbol u). By international agreement, the
reference element is chosen to be the most
abundant type or isotope of carbon, which is
called carbon-12. Its atomic mass is defined to
be exactly twelve atomic mass units, or 12 u. The
relationship between the atomic mass unit and the
kilogram is
2
A portion of the periodic table showing the
atomic number and atomic mass of each element. In
the periodic table it is customary to omit the
symbol u denoting the atomic mass unit.
3
The molecular mass of a molecule is the sum of
the atomic masses of its atoms. Macroscopic
amounts of materials contain large numbers of
atoms or molecules. Even in a small volume of
gas, 1 cm3, for example, the number is enormous.
It is convenient to express such large numbers in
terms of a single unit, the gram-mole, or simply
the mole (symbol mol). One gram-mole of a
substance contains as many particles (atoms or
molecules) as there are atoms in 12 grams of the
isotope carbon-12. 12 grams of carbon-12 contain
6.022 1023 atoms. The number of atoms per mole
is known as Avogadros number NA, after the
Italian scientist Amedeo Avogadro (17761856)
4
(No Transcript)
5
The mass per mole (in g/mol) of a substance has
the same numerical value as the atomic or
molecular mass of the substance (in atomic mass
units).
6
Example 1. The Hope Diamond and the Rosser
Reeves Ruby
The Hope diamond (44.5 carats), which is almost
pure carbon. The Rosser Reeves ruby (138 carats),
which is primarily aluminum oxide (Al2O3). One
carat is equivalent to a mass of 0.200 g.
Determine (a) the number of carbon atoms in the
diamond and (b) the number of Al2O3 molecules in
the ruby.
(a)
m (44.5 carats)(0.200 g)/(1 carat) 8.90
g
7
(b)
m (138 carats)(0.200 g)/(1 carat) 27.6
g.
.
Calculations like those in part (a) reveal that
the Rosser Reeves ruby contains 0.271 mol or
8
Check Your Understanding 1
A gas mixture contains equal masses of the
monatomic gases argon (atomic mass 39.948 u)
and neon (atomic mass 20.179 u). They are the
only gases in the mixture. Of the total number of
atoms, what percentage is neon?
0.664
9
The Ideal Gas Law
An ideal gas is an idealized model for real gases
that have sufficiently low densities.
10
The absolute pressure of an ideal gas is
proportional to the number of molecules or,
equivalently, to the number of moles n of the gas
(P n).
P nT/V.
IDEAL GAS LAW The absolute pressure P of an ideal
gas is directly proportional to the Kelvin
temperature T and the number of moles n of the
gas and is inversely proportional to the volume V
of the gas P R(nT/V). In other words,
where R is the universal gas constant and has the
value of 8.31 J/(molK).
11
The constant term R/NA is referred to as
Boltzmanns constant, in honor of the Austrian
physicist Ludwig Boltzmann (18441906), and is
represented by the symbol k
12
Example 2. Oxygen in the Lungs
In the lungs, the respiratory membrane separates
tiny sacs of air (absolute pressure 1.00 105
Pa) from the blood in the capillaries. These sacs
are called alveoli, and it is from them that
oxygen enters the blood. The average radius of
the alveoli is 0.125 mm, and the air inside
contains 14 oxygen. Assuming that the air
behaves as an ideal gas at body temperature (310
K), find the number of oxygen molecules in one of
the sacs.
.
13
One mole of an ideal gas occupies a volume of
22.4 liters at a temperature of 273 K (0 C) and
a pressure of one atmosphere (1.013 105 Pa).
These conditions of temperature and pressure are
known as standard temperature and pressure (STP).
14
Conceptual Example 3. Beer Bubbles on the Rise
The next time you get a chance, watch the bubbles
rise in a glass of beer. If you look carefully,
youll see them grow in size as they move upward,
often doubling in volume by the time they reach
the surface. Why does a bubble grow as it ascends?
The number of moles does increase as the bubble
rises. Each bubble acts as a nucleation site for
CO2 molecules, so as a bubble moves upward, it
accumulates carbon dioxide from the surrounding
beer and grows larger.
15
Boyles law
A pressure-versus-volume plot for a gas at a
constant temperature is called an isotherm. For
an ideal gas, each isotherm is a plot of the
equation P nRT/V constant/V.
16
Check Your Understanding 2
Consider equal masses of the three monatomic
gases argon (atomic mass 39.948 u), krypton
(atomic mass 83.80 u), and xenon (atomic mass
131.29 u). The pressure and volume of each is the
same. Which gas has the greatest and which the
smallest temperature?
Xenon has the greatest and argon the smallest
temperature.
17
Example 4. Scuba Diving
In scuba diving, a greater water pressure acts on
a diver at greater depths. The air pressure
inside the body cavities (e.g., lungs, sinuses)
must be maintained at the same pressure as that
of the surrounding water otherwise they would
collapse. A special valve automatically adjusts
the pressure of the air breathed from a scuba
tank to ensure that the air pressure equals the
water pressure at all times. The scuba gear
consists of a 0.0150-m3 tank filled with
compressed air at an absolute pressure of 2.02
107 Pa. Assuming that air is consumed at a rate
of 0.0300 m3 per minute and that the temperature
is the same at all depths, determine how long the
diver can stay under seawater at a depth of (a)
10.0 m and (b) 30.0 m.
18
(a)
19
(b) The calculation here is like that in part
(a). Absolute water pressure
is 4.02 105 Pa
Vf 0.754 m3
20
Frenchman Jacques Charles (17461823) discovered
that at a constant pressure, the volume of a
fixed mass (fixed number of moles) of a
low-density gas is directly proportional to the
Kelvin temperature (V T).
Charles law
21
Kinetic Theory of Gases
22
THE DISTRIBUTION OF MOLECULAR SPEEDS
23
KINETIC THEORY
The pressure that a gas exerts is caused by the
collisions of its molecules with the walls of the
container.
24
A gas particle is shown colliding elastically
with the right wall of the container and
rebounding from it.
25
(No Transcript)
26
(No Transcript)
27
Conceptual Example 5. Does a Single Particle
Have a Temperature?
Each particle in a gas has kinetic energy.
Furthermore, the equation
establishes the relationship between the
average kinetic energy per particle and the
temperature of an ideal gas. Is it valid, then,
to conclude that a single particle has a
temperature?
A single gas particle does not have a
temperature.
28
Check Your Understanding 3
The pressure of a monatomic ideal gas is doubled,
while its volume is reduced by a factor of four.
What is the ratio of the new rms speed of the
atoms to the initial rms speed?
29
Example 6. The Speed of Molecules in Air
Air is primarily a mixture of nitrogen N2
(molecular mass 28.0 u) and oxygen O2
(molecular mass 32.0 u). Assume that each
behaves as an ideal gas and determine the rms
speeds of the nitrogen and oxygen molecules when
the temperature of the air is 293 K.
30
(No Transcript)
31
THE INTERNAL ENERGY OF A MONATOMIC IDEAL GAS
The internal energy of a substance is the sum of
the various kinds of energy that the atoms or
molecules of the substance possess. A monatomic
ideal gas is composed of single atoms. These
atoms are assumed to be so small that the mass is
concentrated at a point, with the result that the
moment of inertia I about the center of mass is
negligible.
32
Diffusion
The process in which molecules move from a region
of higher concentration to one of lower
concentration is called diffusion. The host
medium, such as the air or water, is referred to
as the solvent, while the diffusing substance,
like the perfume molecules, is known as the
solute. Relatively speaking, diffusion is a slow
process, even in a gas.
33
Conceptual Example 7. Why Diffusion Is
Relatively Slow
In Example 6 we have seen that a gas molecule has
a translational rms speed of hundreds of meters
per second at room temperature. At such a speed,
a molecule could travel across an ordinary room
in just a fraction of a second. Yet, it often
takes several seconds, and sometimes minutes, for
the fragrance of a perfume to reach the other
side of a room. Why does it take so long?
34
When a perfume molecule diffuses through air, it
makes millions of collisions each second with air
molecules. The speed and direction of motion
change abruptly as a result of each collision.
Between collisions, the perfume molecule moves in
a straight line at a constant speed. Although a
perfume molecule does move very fast between
collisions, it wanders only slowly away from the
bottle because of the zigzag path resulting from
the collisions. It would take a long time for a
molecule to diffuse in this manner across a room.
Usually, however, convection currents are present
and carry the fragrance across the room in a
matter of seconds or minutes.
35
Using diffusion, a transdermal patch delivers a
drug directly into the skin, where it enters
blood vessels. The backing contains the drug
within the reservoir, and the control membrane
limits the rate of diffusion into the skin.
Another way to control the diffusion is to adjust
the concentration of the drug in the reservoir by
dissolving it in a neutral material.
36
(a) Solute diffuses through the channel from the
region of higher concentration to the region of
lower concentration. (b) Heat is conducted along
a bar whose ends are maintained at different
temperatures.
37
FICKS LAW OF DIFFUSION The mass m of solute that
diffuses in a time t through a solvent contained
in a channel of length L and cross-sectional area
A is
where C is the concentration difference
between the ends of the channel and D is the
diffusion constant. SI Unit for the Diffusion
Constant m2/s
38
Example 8. Water Given Off by Plant Leaves
39
Large amounts of water can be given off by
plants. It has been estimated, for instance, that
a single sunflower plant can lose up to a pint of
water a day during the growing season. Figure
14.17 shows a cross-sectional view of a leaf.
Inside the leaf, water passes from the liquid
phase to the vapor phase at the walls of the
mesophyll cells. The water vapor then diffuses
through the intercellular air spaces and
eventually exits the leaf through small openings,
called stomatal pores. The diffusion constant for
water vapor in air is D 2.4 105 m2/s. A
stomatal pore has a cross-sectional area of about
A 8.0 1011 m2 and a length of about L 2.5
105 m. The concentration of water vapor on the
interior side of a pore is roughly C2 0.022
kg/m3, while that on the outside is approximately
C1 0.011 kg/m3. Determine the mass of water
vapor that passes through a stomatal pore in one
hour.
40
(No Transcript)
41
Check Your Understanding 4
The same solute is diffusing through the same
solvent in each case referred to in the table
below, which gives the length and cross-sectional
area of the diffusion channel. In each case, the
concentration difference between the ends of the
diffusion channel is the same. Rank the diffusion
rates (in kg/s) in descending order (largest
first).
c, b, a
42
Concepts Calculations Example 9. The Ideal
Gas Law and Springs
43
There are three identical chambers containing a
piston and a spring whose spring constant is k
5.8 104 N/m. The chamber in part a is
completely evacuated, and the piston just touches
its left end. In this position, the spring is
unstrained. In part b of the drawing, 0.75 mol of
ideal gas 1 is introduced into the chamber, and
the spring compresses by x1 15 cm. In part c,
0.75 mol of ideal gas 2 is introduced into the
chamber, and the spring compresses by x2 24 cm.
Find the temperature of each gas.
P F/A
F kx
P kx/A
44
(No Transcript)
45
Concepts Calculations Example 10. Hydrogen
Atoms in Outer Space
In outer space the density of matter is extremely
low, about one atom per cm3. The matter is mainly
hydrogen atoms (m 1.67 1027 kg) whose rms
speed is 260 m/s. A cubical box, 2.0 m on a side,
is placed in outer space, and the hydrogen atoms
are allowed to enter. (a) What is the magnitude
of the force that the atoms exert on one wall of
the box? (b) Determine the pressure that the
atoms exert. (c) Does outer space have a
temperature, and, if so, what is it?
46
(a)
47
(b)
(c)
48
Problem 22
REASONING AND SOLUTION If the pressure at the
surface is P1 and the pressure at a depth h is
P2, we have that P2 P1 gh. We also know
that P1V1 P2V2. Then,
49
Problem 41
REASONING AND SOLUTION
a. As stated, the time required for the first
solute molecule to traverse a channel of length L
is . Therefore, for
water vapor in air at 293 K, where the diffusion
constant is D2.410-5m2/s , the time t
required for the first water molecule to travel
is
50
b. If a water molecule were traveling at the
translational rms speed for water, the time t it
would take to travel the distance
would be given by
, where, according to Equation 14.6 (
),
. Before we can use the last expression for the
translation rms speed vrms, we must determine the
mass m of a water molecule and the average
translational kinetic energy
Using the periodic table on the inside of the
texts back cover, we find that the molecular
mass of a water molecule is
51
The average translational kinetic energy of water
molecules at 293 K is, according to Equation
14.6,
52
Thus, the time t required for a water molecule to
travel the distance at this speed is
c. In part (a), when a water molecule diffuses
through air, it makes millions of collisions each
second with air molecules. The speed and
direction changes abruptly as a result of each
collision. Between collisions, the water
molecules move in a straight line at constant
speed. Although a water molecule does move very
quickly between collisions, it wanders only very
slowly in a zigzag path from one end of the
channel to the other. In contrast, a water
molecule traveling unobstructed at its
translational rms speed as in part (b), will
have a larger displacement over a much shorter
time. Therefore, the answer to part (a) is much
longer than the answer to part (b)
53
Problem 42
REASONING AND SOLUTION Ficks law of diffusion
gives
54
Problem 44
REASONING AND SOLUTION
a. The average concentration is Cav (1/2) (C1
C2) (1/2)C2 m/V m/(AL), so that
C2 2m/(AL). Fick's law then becomes m
DAC2t/L DA(2m/AL)t/L 2Dmt/L2. Solving for t
yields
b. Substituting into this expression yields
t (2.5 102 m)2/2(1.0 105 m2/s)