Bohrs Third Postulate PowerPoint PPT Presentation
1 / 22
Title: Bohrs Third Postulate
1
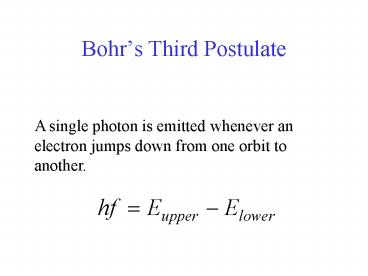
Bohrs Third Postulate
A single photon is emitted whenever an electron
jumps down from one orbit to another.
2
(No Transcript)
3
- Why do astronomers often use the terms color and
temperature interchangeably when referring to
stars? - Why did Bohr assume that the electrons do not
radiate when they are in the allowed orbits?
4
Wave Nature of Matter
5
De Broglie Wavelength
6
Wave-particle duality apply also to matter.
7
De Broglies Hypothesis Applied to Atoms
8
Quantum Mechanics of Atoms
9
The Wave Function and Its Interpolation
10
In quantum mechanics the amplitude of a particle
wave is called the wave function and is given the
symbol Y.
11
If we are dealing only with one photon At any
point the square of the electric field strength
is a measure of the probability that a photon
will be at that location.
12
For a single particle Y2 at a certain point in
space and time represents the probability of
finding the electron at the given position and
time.
13
(No Transcript)
14
Important there is no way to predict where one
electron would hit the screen. We could predict
only probabilities.
15
The main point of this discussion is this if we
treat electrons as if they were waves, then Y
represents the wave amplitude. If we treat them
as particles, then we must treat them on
probabilistic basis.
16
The Heisenberg Uncertainty Principle
17
(No Transcript)
18
The act of observing produces a significant
uncertainty in either the position or the
momentum of electron.
19
(No Transcript)
20
Position uncertainty of a baseball
What is the uncertainty in position, imposed by
the uncertainty principle, of a 150-g baseball
thrown at 42-1 m/s? Should the umpire be
concerned? Can he use Heisenberg as an excuse?
21
Particle in a Box
22
Baby-Quiz
- If all objects emit radiation, why dont we see
most of them in the dark? - Suppose you were a nineteenth-century scientist
who had just discovered a new phenomenon known as
Zeta rays. What experiment could you perform to
define if Zeta rays are charged particles or e/m
waves? Could this experiment distinguish between
neutral particles and an e/m wave? - If a metal surface is illuminated by light at a
single frequency, why dont all the
photoelectrons have the same kinetic energy when
they leave the metals surface? - What property of the emitted electrons depends on
the intensity of incident light?What property of
the emitted photoelectrons depends on the
frequency of incident light?