Halogen Compound - PowerPoint PPT Presentation
1 / 47
Title: Halogen Compound
1
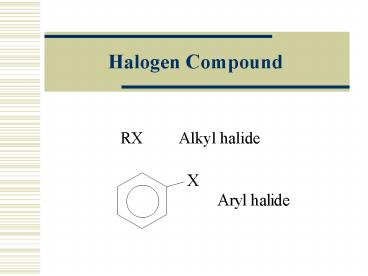
Halogen Compound
RX Alkyl halide
2
A. Preparation
- 1. Halogenation
3
A. Preparation
- 2. With HX
HX is come from NaX conc. H2SO4
4
A. Preparation
- 3. From alcohol
5
A. Preparation
- 3. From alcohol cont.
- We rarely start with PCl3, PBr3 or PI3, since
they are easily hydrolysed by moisture in air.
Instead, they are made in situ, using red
phosphorus in the alcohol and halogen is put into
the reaction flask as the reaction proceeds.
6
A. Preparation
- 3. From alcohol cont.
ROH HX ? RX H2O
(usually HBr, HI BUT RCl cannot be prepared by
this method)
Pyridine (as solvent)
ROH SOCl2 ? RCl SO2
HCl
(This product is most easily purified)
7
A. Preparation
- 3. From alcohol cont.
For aryl halide
8
A. Preparation
- 4. Diazonium coupling (for aryl halide only)
- Formation of a diazonium salt
9
A. Preparation
- 4. Diazonium coupling cont.
From diazonium salt, you can make the following
aryl halide
10
A. Preparation
- 4. Diazonium coupling cont.
From diazonium salt, you can make the following
aryl halide
11
B. Physical properties
- It has a little higher boiling point than
corresponding alkane of comparable molecular
mass. This is due to the dipole-dipole
attraction between the molecules as they are
polar. - CH3Cl, CH3Br and C2H5Cl are gases in room
temperature while other members are liquids.
Chlorobenzene is colourless liquid. All alkyl and
aryl halides are insoluble in water due to the
inability to form extensive H-bond with water
molecules.
12
C. Chemical Properties
- Nucleophilic Substitution
- Halogen compounds are polar compounds. The
electron deficient carbon attach to the halogen
is susceptible to the attack of an electron rich
species (nucleophile) and undergo nucleophilic
substitution.
13
C. Chemical Properties
- Nucleophilic Substitution cont.
- Alkyl halide is very reactive because the C-X
bond is very polar while in aryl halide, the
reaction proceeds with difficulty. It is because
the p-orbital of X overlap with the pi-electron
of the ring, causing a delocalization of
electron. The C-X bond is thus less polar and
stronger (possess double bond character).
14
C. Chemical Properties
15
Chemical properties
- Lateral overlapping of the p-orbital of X with
the pi molecular orbital of benzene
delocalization of electron throughout the whole
structure
16
C. Chemical Properties
- a. Hydrolysis
Side product alkene (From dehydrohalogenation)
Do you remember this reaction?
17
C. Chemical Properties
- a. Hydrolysis cont.
- Side product alkene (From dehydrohalogenation)
18
C. Chemical Properties
- a. Hydrolysis cont.
- For phenol industrial process
- (not important)
19
C. Chemical Properties
- Mechanism - Bimolecular Nucleophilic Substitution
SN2
Press
Transition state (trigonal bipyramidal)
20
C. Chemical Properties
- Bimolecular
- Molecularity refers to the number of species
that are undergoing bond-making and / or
bond-breaking process in the rate determining
step. - Rate k alkyl halide1 OH-1
- ? Second order reaction
21
C. Chemical Properties
Transition state
22
C. Chemical Properties
- Mechanism - Unimolecular Nucleophilic
Substitution SN1
Press
23
C. Chemical Properties
- Unimolecular
- In rate determinating step, only involve one
molecule - Rate k alkyl halide1 OH-0
24
C. Chemical Properties
intermediate
25
C. Chemical Properties
- Stability of carbonium ion
26
C. Chemical Properties - Factors affecting choice
of mechanism
- Structure of alkyl halide
3ry 2ry 1ry CH3
Use of 3ry alkyl halide favour SN1 since
- Alkyl group is electron-donating which helps
- to stablilise the carbonium ion, thus lower the
EA.
27
C. Chemical Properties Factors affecting choice
of mechanism
- Use of 3ry alkyl halide favour SN1 since
- Alkyl groups hinder the approach of a nucleophile
- (OR steric crowding at T.S. would destabilise a
bimolecular transition state, thus increase the
EA.)
is less stable than
Favour SN2
Not favour SN2
(Why not consider the steric crowding at
carbonium ion?)
28
C. Chemical Properties Factors affecting choice
of mechanism
- Solvent
- Highly polar (ionising) solvent favour SN1
(because forming ion in 1st step) - Polar solvent aqueous, THF
- Less polar solvent alcoholic
29
C. Chemical Properties Factors affecting choice
of mechanism
- Choice of nucleophile
- Strong nucleophile in high conc. favour SN2
while weak nucleophile in dilute solution favour
SN1. - Strong nucleophile Weak nucleophile
- OH- H2O
- NH2- NH3
- CN- HCN
- RO- ROH
- 4. Presence of Ag ion favour SN1
30
C. Chemical Properties
- Effect of halogen
- Since the electronagativity of halogen decreased
down the group, C-Cl bond is more polar than the
others. Hence, the carbon join to Cl is the most
electron deficient, so the carbon in RCl in most
susceptible to the attack of nucleophile. - The bond strength is also important in
determining the rate since bond strength - decreased rapidly from C-Cl to C-I bond, the
reaction rate decreases in the order - R I gt R Br gt R Cl
31
C. Chemical Properties Summary of SN reaction -
Press
32
C. Chemical Properties Summary of SN reaction -
33
C. Chemical Properties Summary of SN reaction -
34
C. Chemical Properties
- b. Formation of amine
- If RX is in excess, further reaction is expected
since RNH2 is an even stronger nucleophile.
35
C. Chemical Properties
- b. Formation of amine (cont.)
- RX RNH2 ? R2NH HX
- RX R2NH ? R3N HX
- RX R3N ? R4N X-
Quarternary ammonium salt
36
C. Chemical Properties
- b. Formation of amine (cont.)
This reaction is not important because under
normal condition aryl halide is very difficult to
have nucleophilic substitution rx (Why?)
37
C. Chemical Properties
- c. Formation of nitrile
38
C. Chemical Properties
- d. Formation of ether (Williamsons synthesis)
RX is usually 1ry alkyl halide should NOT be 2ry
and 3ry. (Why?)
39
C. Chemical Properties
- e. Formation of ester
- RX RCOO- Ag ? RCOOR AgX
- (Why do we use Ag compound?)
40
C. Chemical Properties
- Elimination
- Dehydrohalogenation
- That is, dehydrohalogenation reaction when alkyl
halide is heated with a strong base using a
relative non-polar solvent.
41
C. Chemical Properties
- Elimination Dehydrohalogenation (cont.)
- Note that both base and nucleophile are
electron rich species, hence both elimination and
nucleophilic reaction would occur at the same
time unless the reaction conditions are carefully
chosen. - Normally, elimination reaction occurs at high
temperature, alcoholic medium (relatively
non-polar) and the alkyl halide is highly
branched (e.g. - tertiary or secondary).
42
C. Chemical Properties
- Electrophilic substitution (Disubstitution is
O.S.)
minor
major
43
Chemical properties
- 4. Formation of Grignard Reagent
excess
44
Chemical properties
- 5. Reaction with Grignard reagent
alkane
What kind of reaction is this ?
How do you prepare
45
Chemical properties
- Wurtz Reaction
- Wurtz Fittig Reaction
46
C. Chemical Properties
- Reduction
47
D. Uses of halogen-compound
- 1. As solvents in dry-cleaning
- 2. As raw materials in the manufacture of
poly(chloroethene) and poly(tetrafluoroethene).