IONIC COMPOUNDS Chapter 5 - PowerPoint PPT Presentation
1 / 77
Title:
IONIC COMPOUNDS Chapter 5
Description:
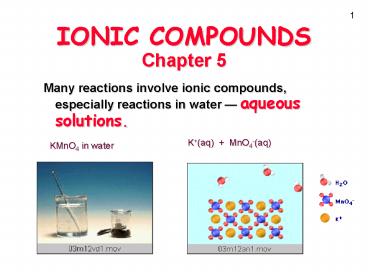
Chapter 5 Many reactions involve ionic compounds, especially reactions in water aqueous solutions. K+(aq) + MnO4-(aq) KMnO4 in water An Ionic Compound, CuCl2, in ... – PowerPoint PPT presentation
Number of Views:134
Avg rating:3.0/5.0
Title: IONIC COMPOUNDS Chapter 5
1
IONIC COMPOUNDSChapter 5
- Many reactions involve ionic compounds,
especially reactions in water aqueous solutions.
KMnO4 in water
2
An Ionic Compound, CuCl2, in Water
3
Aqueous Solutions
- How do we know ions are present in aqueous
solutions? - The solutions conduct electricity!
- They are called ELECTROLYTES
- HCl, MgCl2, and NaCl are strong electrolytes.
They dissociate completely (or nearly so) into
ions.
4
Aqueous Solutions
- HCl, MgCl2, and NaCl are strong electrolytes.
They dissociate completely (or nearly so) into
ions.
5
Aqueous Solutions
- Acetic acid ionizes only to a small extent, so it
is a weak electrolyte. - CH3CO2H(aq) ---gt CH3CO2-(aq) H(aq)
6
Aqueous Solutions
- Acetic acid ionizes only to a small extent, so it
is a weak electrolyte. - CH3CO2H(aq) ---gt CH3CO2-(aq) H(aq)
7
Aqueous Solutions
- Some compounds dissolve in water but do not
conduct electricity. They are called
nonelectrolytes.
Examples include sugar ethanol ethylene glycol
8
Water Solubility of Ionic Compounds
If one ion from the Soluble Compd. list is
present in a compound, the compound is water
soluble.
Screen 5.4 Figure 5.1
Guidelines to predict the solubility of ionic
compounds
9
Water Solubility of Ionic Compounds
- Common minerals are often formed with anions that
lead to insolubility - sulfide fluoride
- carbonate oxide
10
ACIDS
An acid -------gt H in water
- Some strong acids are
- HCl hydrochloric
- H2SO4 sulfuric
- HClO4 perchloric
- HNO3 nitric
11
ACIDS
An acid -------gt H in water
- HCl(aq) ---gt H(aq) Cl-(aq)
12
The Nature of Acids
13
Weak Acids
- WEAK ACIDS weak electrolytes
- CH3CO2H
- acetic acid
- H2CO3 carbonic acid
- H3PO4 phosphoric acid
- HF
- hydrofluoric acid
14
ACIDS
- Nonmetal oxides can be acids
- CO2(aq) H2O(liq) ---gt H2CO3(aq)
- SO3(aq) H2O(liq) ---gt H2SO4(aq)
- and can come from burning coal and oil.
15
BASESsee Screen 5.9 and Table 5.2
Base ---gt OH- in water
- NaOH(aq) ---gt Na(aq) OH-(aq)
NaOH is a strong base
16
Ammonia, NH3An Important Base
17
BASES
- Metal oxides are bases
- CaO(s) H2O(liq)
- --gt Ca(OH)2(aq)
CaO in water. Indicator shows solution is basic.
18
Know the strong acids bases!
19
Net Ionic Equations
- Mg(s) 2 HCl(aq) --gt H2(g) MgCl2(aq)
- We really should write
- Mg(s) 2 H(aq) 2 Cl-(aq) ---gt
H2(g) Mg2(aq) 2 Cl-(aq)
The two Cl- ions are SPECTATOR IONS they do not
participate. Could have used NO3-.
20
Net Ionic Equations
- Mg(s) 2 HCl(aq) --gt H2(g)
MgCl2(aq) - Mg(s) 2 H(aq) 2 Cl-(aq) ---gt H2(g)
Mg2(aq) 2 Cl-(aq)
We leave the spectator ions out Mg(s) 2
H(aq) ---gt H2(g) Mg2(aq)
to give the NET IONIC EQUATION
21
Chemical Reactions in WaterSections 5.2
5.4-5.6CD-ROM Ch. 5
- We will look at EXCHANGE REACTIONS
Pb(NO3) 2(aq) 2 KI(aq) ----gt PbI2(s) 2
KNO3 (aq)
The anions exchange places between cations.
22
Precipitation Reactions
- The driving force is the formation of an
insoluble compound a precipitate. - Pb(NO3)2(aq) 2 KI(aq) -----gt
- 2 KNO3(aq) PbI2(s)
- Net ionic equation
- Pb2(aq) 2 I-(aq) ---gt PbI2(s)
23
Acid-Base Reactions
- The driving force is the formation of water.
- NaOH(aq) HCl(aq) ---gt
- NaCl(aq) H2O(liq)
- Net ionic equation
- OH-(aq) H(aq) ---gt H2O(liq)
- This applies to ALL reactions of STRONG acids and
bases.
24
Acid-Base Reactions CCR, page 162
25
Acid-Base Reactions
- A-B reactions are sometimes called
NEUTRALIZATIONS because the solution is neither
acidic nor basic at the end. - The other product of the A-B reaction is a SALT,
MX. - HX MOH ---gt MX H2O
- Mn comes from base Xn- comes from acid
- This is one way to make ionic compounds!
26
Gas-Forming Reactions
- This is primarily the chemistry of metal
carbonates. - CO2 and water ---gt H2CO3
- H2CO3(aq) Ca2 ---gt
- 2 H(aq) CaCO3(s) (limestone)
- Adding acid reverses this reaction.
- MCO3 acid ---gt CO2 salt
27
Gas-Forming Reactions
- CaCO3(s) H2SO4(aq) ---gt
- 2 CaSO4(s) H2CO3(aq)
- Carbonic acid is unstable and forms CO2 H2O
- H2CO3(aq) ---gt CO2 (g) water
- (Antacid tablet has citric acid NaHCO3)
28
See also Gas Forming Reactions in Biological
Systems Three of the pioneers in working out the
roles of NO forming reactions shared a Nobel
Prize in 1988 for their discoveries.
29
Quantitative Aspects of Reactions in
SolutionSections 5.8-5.10
30
Terminology
- In solution we need to define the -
- SOLVENT
- the component whose physical state
is preserved when
solution forms - SOLUTE
- the other solution component
31
Concentration of Solute
- The amount of solute in a solution is given by
its concentration.
Concentration (M)
32
1.0 L of water was used to make 1.0 L of
solution. Notice the water left over.
CCR, page 177
33
PROBLEM Dissolve 5.00 g of NiCl26 H2O in
enough water to make 250 mL of solution.
Calculate molarity.
Step 1 Calculate moles of NiCl26H2O
Step 2 Calculate molarity
NiCl26 H2O 0.0841 M
34
The Nature of a CuCl2 SolutionIon Concentrations
- CuCl2(aq) --gt
- Cu2(aq) 2 Cl-(aq)
- If CuCl2 0.30 M, then
- Cu2 0.30 M
- Cl- 2 x 0.30 M
35
USING MOLARITY
- What mass of oxalic acid, H2C2O4, is required to
make 250. mL of a 0.0500 M solution? - Because
Conc (M) moles/volume mol/V - this means that
36
USING MOLARITY
What mass of oxalic acid, H2C2O4, is required to
make 250. mL of a 0.0500 M solution?
moles MV
- Step 1 Calculate moles of acid required.
- (0.0500 mol/L)(0.250 L) 0.0125 mol
- Step 2 Calculate mass of acid required.
- (0.0125 mol )(90.00 g/mol) 1.13 g
37
Preparing Solutions
- Weigh out a solid solute and dissolve in a given
quantity of solvent. - Dilute a concentrated solution to give one that
is less concentrated.
38
PROBLEM You have 50.0 mL of 3.0 M NaOH and you
want 0.50 M NaOH. What do you do?
- Add water to the 3.0 M solution to lower its
concentration to 0.50 M - Dilute the solution!
39
PROBLEM You have 50.0 mL of 3.0 M NaOH and you
want 0.50 M NaOH. What do you do?
But how much water do we add?
40
PROBLEM You have 50.0 mL of 3.0 M NaOH and you
want 0.50 M NaOH. What do you do?
- How much water is added?
- The important point is that ---gt
41
PROBLEM You have 50.0 mL of 3.0 M NaOH and you
want 0.50 M NaOH. What do you do?
- Amount of NaOH in original solution
- M V
- (3.0 mol/L)(0.050 L) 0.15 mol NaOH
- Amount of NaOH in final solution must also 0.15
mol NaOH - 0.15/Volume of final solution 0.5 M/ 1 L
- Volume of final solution
- (0.15 mol NaOH)(1 L/0.50 mol) 0.30 L
- or 300 mL
42
PROBLEM You have 50.0 mL of 3.0 M NaOH and you
want 0.50 M NaOH. What do you do?
- Conclusion
- add 250 mL of water to 50.0 mL of 3.0 M NaOH to
make 300 mL of 0.50 M NaOH.
43
Preparing Solutions by Dilution
- A shortcut
- Cinitial Vinitial Cfinal Vfinal
44
The pH Scale
- pH log (1/ H)
- - log H
- Remember log a b if 10ba
- In a neutral solution, H OH- 1.00
x 10-7 M at 25 oC - pH - log H -log (1.00 x 10-7)
- (-7) - 7
- See CD Screen 5.17 for a tutorial
45
pH, a Concentration Scale
- pH a way to express acidity -- the concentration
of H in solution.
Low pH high H
High pH low H
Acidic solution pH lt 7 Neutral pH 7 Basic
solution pH gt 7
46
H and pH
- If the H of soda is 1.6 x 10-3 M, the pH is
____? - Because pH - log H
- then
- pH - log (1.6 x 10-3)
- pH - (-2.80)
- pH 2.80
Whats the origin of the name of the soda 7up ?
47
ACID-BASE REACTIONSTitrations
- H2C2O4(aq) 2 NaOH(aq) ---gt
- acid base
- Na2C2O4(aq) 2 H2O(liq)
- Carry out this reaction using a TITRATION.
48
Setup for titrating an acid with a base
CCR, page 186
49
Titration
- 1. Add solution from the buret.
- 2. Reagent (base) reacts with compound (acid) in
solution in the flask. - 3. Indicator shows when exact stoichiometric
reaction has occurred. - 4. Net ionic equation
- H OH- --gt H2O
- 5. At equivalence point
- moles H moles OH-
50
LAB PROBLEM 1 Standardize a solution of NaOH
i.e., accurately determine its concentration.
- 1.065 g of H2C2O4 (oxalic acid) requires 35.62
mL of NaOH for titration to an equivalence point.
What is the concentration of the NaOH?
51
1.065 g of H2C2O4 (oxalic acid) requires 35.62 mL
of NaOH for titration to an equivalence point.
What is the concentration of the NaOH?
- Step 1 Calculate amount of H2C2O4
Step 2 Calculate amount of NaOH reqd
52
1.065 g of H2C2O4 (oxalic acid) requires 35.62 mL
of NaOH for titration to an equivalence point.
What is the concentration of the NaOH?
- Step 1 Calculate amount of H2C2O4
- 0.0118 mol acid
- Step 2 Calculate amount of NaOH reqd
- 0.0236 mol NaOH
- Step 3 Calculate concentration of NaOH
NaOH 0.663 M
53
LAB PROBLEM 2 Use standardized NaOH to
determine the amount of an acid in an unknown.
- Apples contain malic acid, C4H6O5.
- C4H6O5(aq) 2 NaOH(aq) ---gt
- Na2C4H4O5(aq) 2 H2O(liq)
- 76.80 g of apple requires 34.56 mL of 0.663 M
NaOH for titration. What is weight of malic
acid?
54
76.80 g of apple requires 34.56 mL of 0.663 M
NaOH for titration. What is weight of malic
acid?
- Step 1 Calculate amount of NaOH used.
- C V (0.663 M)(0.03456 L)
- 0.0229 mol NaOH
- Step 2 Calculate amount of acid titrated.
0.0115 mol acid
55
76.80 g of apple requires 34.56 mL of 0.663 M
NaOH for titration. What is weight of malic
acid?
Step 1 Calculate amount of NaOH used.
0.0229 mol NaOH Step 2 Calculate amount
of acid titrated 0.0115 mol acid
- Step 3 Calculate mass of acid titrated.
56
76.80 g of apple requires 34.56 mL of 0.663 M
NaOH for titration. What is weight of malic
acid?
- Step 1 Calculate amount of NaOH used.
- 0.0229 mol NaOH
- Step 2 Calculate amount of acid titrated
- 0.0115 mol acid
- Step 3 Calculate mass of acid titrated.
- 1.54 g acid
Step 4 Calculate malic acid.
57
pH and H
- If the pH of Coke is 3.12, it is ____________.
- Because pH - log H then
- log H - pH
- Take antilog and get
- H 10-pH
- H 10-3.12 7.6 x 10-4 M
58
SOLUTION STOICHIOMETRYGas-forming reactions
- Zinc reacts with acids to produce H2 gas.
- Have 10.0 g of Zn
- What volume of 2.50 M HCl is needed to convert
the Zn completely?
59
GENERAL PLAN FOR STOICHIOMETRY CALCULATIONS
60
Zinc reacts with acids to produce H2 gas. If you
have 10.0 g of Zn, what volume of 2.50 M HCl is
needed to convert the Zn completely?
- Step 1 Write the balanced equation
- Zn(s) 2 HCl(aq) --gt ZnCl2(aq) H2(g)
- Step 2 Calculate amount of Zn
Step 3 Use the stoichiometric factor
61
Zinc reacts with acids to produce H2 gas. If you
have 10.0 g of Zn, what volume of 2.50 M HCl is
needed to convert the Zn completely?
- Step 3 Use the stoichiometric factor
Step 4 Calculate volume of HCl reqd
62
EXCHANGE Precipitation Reactions
EXCHANGE Acid-Base Reactions
EXCHANGE Gas-Forming Reactions
REACTIONS
REDOX REACTIONS
63
REDOX REACTIONS
- Redox reactions are characterized by ELECTRON
TRANSFER between an electron donor and electron
acceptor. - Transfer leads to
- 1. increase in oxidation number of some element
OXIDATION - 2. decrease in oxidation number of some element
REDUCTION
64
REDOX REACTIONS
- Cu(s) 2 Ag(aq)
- ---gt Cu2(aq) 2 Ag(s)
- In all reactions if something has been oxidized
then something has also been reduced
65
Why Study Redox Reactions
Batteries
Corrosion
Manufacturing metals
Fuels
66
OXIDATION NUMBERS
- The electric charge an element APPEARS to have
when electrons are counted by some arbitrary
rules - 1. Each atom in free element has ox. no. 0.
- Zn O2 I2 S8
- 2. In simple ions, ox. no. charge on ion.
- -1 for Cl-
- 2 for Mg2
67
OXIDATION NUMBERS
- 3. F always has an oxidation number of -1 when
forming compounds with other elements. - 4. Cl, Br and I have oxidation numbers of -1 when
forming compounds with other elements, except
when combined with oxygen and fluorine. - 5a. O has ox. no. -2
- (except in peroxides in H2O2, O -1)
68
OXIDATION NUMBERS
- 5b. Ox. no. of H 1
- (except when H is associated with a metal as in
NaH where it is -1) - 6. Algebraic sum of oxidation numbers
- 0 for a compound
- overall charge for an ion
69
OXIDATION NUMBERS
- NH3 N
- ClO- Cl
- H3PO4 P
- MnO4- Mn
- Cr2O72- Cr
- C3H8 C
70
Recognizing a Redox Reaction
- Corrosion of aluminum
- 2 Al(s) 3 Cu2(aq) --gt 2 Al3(aq) 3 Cu(s)
- Al(s) --gt Al3(aq) 3 e-
- Ox. no. of Al increases as e- are donated by the
metal. - Therefore, Al is OXIDIZED
- Al is the REDUCING AGENT in this balanced
half-reaction.
71
Recognizing a Redox Reaction
- Corrosion of aluminum
- 2 Al(s) 3 Cu2(aq) --gt 2 Al3(aq) 3 Cu(s)
- Cu2(aq) 2 e- --gt Cu(s)
- Ox. no. of Cu decreases as e- are accepted by the
ion. - Therefore, Cu is REDUCED
- Cu is the OXIDIZING AGENT in this balanced
half-reaction.
72
Recognizing a Redox Reaction
- Notice that the 2 half-reactions add up to give
the overall reaction if we use 2 moles of Al
and 3 moles of Cu2. - 2 Al(s) --gt 2 Al3(aq) 6 e-
- 3 Cu2(aq) 6 e- --gt 3 Cu(s)
- --------------------------------------------------
--------- - 2 Al(s) 3 Cu2(aq) ---gt 2 Al3(aq) 3 Cu(s)
- Final eqn. is balanced for mass and charge.
73
Common Oxidizing and Reducing AgentsSee Table
5.4
Cu HNO3 --gt Cu2 NO2
2 K 2 H2O --gt 2 KOH H2
74
Recognizing a Redox ReactionSee Table 5.4
Reaction Type
Oxidation
Reduction
- In terms of oxygen gain loss
- In terms of halogen gain loss
- In terms of electrons loss gain
75
Examples of Redox Reactions
Metal halogen 2 Al 3 Br2 ---gt Al2Br6
76
Examples of Redox Reactions
Nonmetal (P) Oxygen
Metal (Mg) Oxygen
77
Examples of Redox Reactions
Metal acid Mg HCl Mg reducing agent H
oxidizing agent
Metal acid Cu HNO3 Cu reducing agent HNO3
oxidizing agent