Molecular Formulas - PowerPoint PPT Presentation
1 / 17
Title:
Molecular Formulas
Description:
Valence Electrons ... for the Main Group elements, the valence electrons are the s and p-electrons in ... Distribute remaining valence electrons so that all ... – PowerPoint PPT presentation
Number of Views:111
Avg rating:3.0/5.0
Title: Molecular Formulas
1
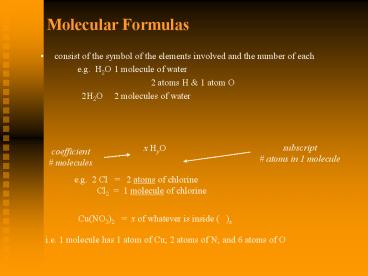
Molecular Formulas
- consist of the symbol of the elements involved
and the number of each - e.g. H2O 1 molecule of water
- 2 atoms H 1 atom O
- 2H2O 2 molecules of water
subscript atoms in 1 molecule
x HyO
coefficient molecules
e.g. 2 Cl 2 atoms of chlorine
Cl2 1 molecule of chlorine
Cu(NO3)2 x of whatever is inside ( )x
i.e. 1 molecule has 1 atom of Cu 2 atoms of N
and 6 atoms of O
2
Valence Electrons
determine the chemical-character of an
element---these are the electrons gained, lost,
or shared in a chemical reaction. for the Main
Group elements, the valence electrons are the s
and p-electrons in the outermost shell (equals
the group ).
use dots to represent valence electrons
for the transition metals, the valence electrons
are those in the outermost ns and (n-1)d subshells
3
Lewis Dot Structures
pairs of dots (lines) between atoms are
bonds. multiple pairs of dots represent multiple
bonds. valence electrons not involved in bonds
are usually in pairs.
4
Covalent Bonding
- involves the sharing of valence electrons between
atoms - separation of nuclei is balance between
electron-nuclear attraction, and
electron-electron nuclear-nuclear repulsions - OCTET RULE
- each atom in a molecule attempts to bond such
that it achieves a Noble Gas configuration - F F N N
- only 2 electrons per covalent bond can have more
than one bond between nuclei
. . . . . . . .
5
Writing Lewis Dot Structures
- Write skeleton structure for molecule
- element with the least negative electron affinity
at center - use formula a hint
- Hs always, and Halides most often, terminal
(NEVER form multiple bonds) - Oxygens do not like to bond together (with a few
exceptions) - Determine number of valence electrons in molecule
- cations () subtract electrons
- anions (-) add electrons
- From this total, subtract 2 electrons for each
bond in skeleton structure - Distribute remaining valence electrons so that
all atoms have complete octet - may need to introduce multiple bonds
6
Writing Lewis Dot Structures Example
SO3
- Write skeleton structure for molecule
- element with the least negative electron affinity
at center - use formula a hint
- Hs always, and Halides most often, terminal
(NEVER form multiple bonds) - Oxygens do not like to bond together (with a few
exceptions)
O S O O
7
Writing Lewis Dot Structures Example
SO3
- Determine number of valence electrons in molecule
- cations () subtract electrons
- anions (-) add electrons
Oxygen 6 x 3 18 e- Sulfur 6 x 1 6
e- Total 24 e-
O S O O
8
Writing Lewis Dot Structures Example
SO3
- From this total, subtract 2 electrons for each
bond in skeleton structure
Oxygen 6 x 3 18 e- Sulfur 6 x 1 6
e- Total 24 e- - Bonds 2
x 3 6 e- Electrons Left 18 e-
O S O O
9
Writing Lewis Dot Structures Example
SO3
- Distribute remaining valence electrons so that
all atoms have complete octet - may need to introduce multiple bonds
Oxygen 6 x 3 18 e- Sulfur 6 x 1 6
e- Total 24 e- - Bonds 2
x 3 6 e- Electrons Left 18 e-
wants 6 more electrons
wants 2 more electrons
O S O O
wants 6 more electrons
wants 6 more electrons
There is a need of 20 electrons, but there are
only 18 left, so 2 must be shared---double bond.
10
Writing Lewis Dot Structures Example
SO3
- Distribute remaining valence electrons so that
all atoms have complete octet - may need to introduce multiple bonds
- Oxygen 6 x 3 18 e-
- Sulfur 6 x 1 6 e-
- Total 24 e-
- - Bonds 2 x 3 6 e-
- Electrons Left 18 e-
- - Bond 2 x 1 2 e-
- Electrons Left 16 e-
wants 6 more electrons
wants 0 more electrons
O S O O
wants 4 more electrons
wants 6 more electrons
There is a need of 16 electrons, and there are
16 left, so distribute as lone pairs.
11
Writing Lewis Dot Structures Example
SO3
- Distribute remaining valence electrons so that
all atoms have complete octet - may need to introduce multiple bonds
- Oxygen 6 x 3 18 e-
- Sulfur 6 x 1 6 e-
- Total 24 e-
- - Bonds 2 x 3 6 e-
- Electrons Left 18 e-
- - Bond 2 x 1 2 e-
- Electrons Left 16 e-
O S O O
12
Exceptions to Octet Rule
- Hydrogen 2 electrons
- Group IIIA elements like 6 electrons e.g. BF3
- electron deficient or incomplete octets
- Elements of atomic number 14 and greater MAY have
expanded octets (10 or 12 e-s)
13
(No Transcript)
14
Formal Charges
- O
- O N OH
- simple means of bookkeeping
- the and - signs do not necessarily indicate
actual charges - negative charge on more electronegative atom
- sum of Formal Charges charge on molecule or ion
- Electroneutrality principle formal Charges are
to be avoided whenever possible - e.g. draw 2 structures for CHN, which actually
exists? - H C N H N C
F.C. valence electrons - nonbonding electrons
- bonds
.. ..
.. ..
N 5 - 0 - 4 1 O 6 - 6- 1 -1
15
Bond Lengths
- distance between adjacent nuclei
- H H H H
- H C C H C C
H C C H - H H H H
- ethane ethylene
acetylene - Trends
- bond lengths shorten on going from single to
double to triple bond (more electrons between
nuclei) - little change in single bond lengths (similar
bond types have similar bond lengths)
0.133nm
0.110nm
0.120nm
0.154nm
0.109nm
16
Resonance
- in many cases there are multiple equivalent
places to put multiple bonds - O
O - e.g. HNO3 O N OH
O N OH - note all N-O bond lengths are identical but
neither single nor double. - if 2 or more Lewis structures exist using the
same backbone, then resonance is said to exist - the individual structures contributing
structures - the molecule itself is a resonance hybrid of the
contributing structures - represent hybrid by drawing contributing
structures and separating them by a - the resonance hybrid is a blend (average) of the
contributing structures
O -
O N OH
-
-
-
bond lengths equivalent to a 1 1/2 bond
17
(No Transcript)