Matter and Measurement - PowerPoint PPT Presentation
Title:
Matter and Measurement
Description:
N2(g) 3 H2(g) -- 2 NH3(g) Chemical Equilibrium ... N2(g) 3H2(g) 2NH3(g) The forward and reverse directions 'oppose' ... PCl5(g) PCl3(g) Cl2 (g) K = 2.15 ... – PowerPoint PPT presentation
Number of Views:145
Avg rating:3.0/5.0
Title: Matter and Measurement
1
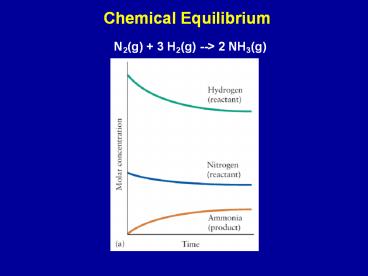
Chemical Equilibrium
N2(g) 3 H2(g) --gt 2 NH3(g)
2
- Many reactions do not go to completion - under
the given conditions it is possible that not all
of the reactants are consumed. - Instead the extent of the reaction is determined
by the equilibrium point. - A B --gt C D
- As the concentrations of C and D increase, C and
D could react to form A and B - the REVERSE
reaction.
N2(g) 3H2(g) 2NH3(g)
3
- The forward and reverse directions oppose one
another. - At some point in time, the rate of the forward
reaction will equal the rate of the reverse
reaction - this point corresponds to EQUILIBRIUM. - Hence, when equilibrium has been reached, the
concentration of A, B, C and D stay constant, as
long as the conditions are held the same. - At the equilibrium point A and B combine to form
C and D C and D combine to form A and B but
both occur at the same rate. - There is no NET change in the concentrations of
A, B, C D.
4
Equilibrium
- When opposing forces acting on a system are equal
in magnitude, the system is said to be in a state
of equilibrium. - A dynamic equilibrium is one at which changes to
the system do occur at the microscopic level, but
at the macroscopic level these changes are not
observed. - In general Processes not at equilibrium will act
or react to reach equilibrium.
5
(No Transcript)
6
(No Transcript)
7
- Characteristics of equilibrium
- 1) The attainment of equilibrium is spontaneous
i.e. it is a natural tendency - 2) At equilibrium there is no macroscopic
evidence of any changes in the system - 3) A dynamic balance is established between
opposing forces - 4) Equilibrium is reached from either direction
8
- The Equilibrium Expression
K (constant)
K is called the EQUILIBRIUM CONSTANT
Note K has a fixed value for a particular
reaction and varies with temperature
9
- If all reactants and products are gases, the
relationship between the partial pressures of all
gases at equilibrium is
If all reactants and products are in solution,
the relationship between the concentrations of
all species at equilibrium is
Where X is the concentration (example molarity)
of species X at equilibrium
Homogenous reactions reactants and products in
the same phase
10
- Heterogeneous reaction reactants and products
are not in the same phase
a A(aq) b B(aq) c C(aq) d D(g)
11
K is a dimensionless quantity. 2A(g)
B(g) K PB/P2A is actually
PB/Pref
K
(PA/Pref)2
Pref is set to 1 atm K is dimensionless For
solutions, if concentration is M Aref 1M
12
HCl(aq) H(aq) Cl-(aq)
H Cl-
K
K 107 at 25oC
HCl
CH3COOH(aq) H(aq) CH3COO-(aq)
K 10-5 at 25oC
13
The reaction of SO2(g) and O2(g) forming SO3(g)
Equilibrium can be reached for different partial
pressures of SO2, O2, and SO3, depending on the
starting conditions, but at 25oC, the value of K
is the same.
14
- The Magnitude of the Equilibrium Constant
- The magnitude of the equilibrium constant reveals
the extent to which the reaction will proceed in
the desired direction.
Reactions that have K values gt 1 are favored in
the direction written i.e. forward
direction. Reactions that have K values lt 1 are
favored in the reverse direction Reactions for
which K is near I have substantial amounts of
both reactants and products when equilibrium is
established.
15
(No Transcript)
16
- Applying the Equilibrium Expression to Gas Phase
Reactions
What are the equilibrium partial pressures of all
three gases in a closed container containing only
PCl5 at 0.100 atm and held at 250oC? According
to the ideal gas laws, the partial pressures of
gases is proportional to the number of moles of
each gas, as long as the volume and temperature
are kept fixed. The stoichiometry of this
reaction is 1 1 1
17
If the partial pressure of PCl5 decreases by x at
equilibrium, the partial pressures of PCl3 and
Cl2 increases by x at equilibrium.
At equilibrium
18
x2 2.15 (0.100-x) x2 2.15x - 0.215 0
- This is a quadratic equation of the form
- ax2 bx c 0
- and the solution of this equation is of the form
19
Using this expression and solving for x, the
roots of the equation are x 0.0957 and -2.25
atm. At equilibrium, the partial pressures of
Cl2 and PCl3 are 0.0957 atm, and that of PCl5 is
(0.100 - 0.0957) 0.004atm
20
The three gases are introduced into a container
at partial pressures of 3.6 atm NO2, 5.1 atm N2O
and 8.0atm O2 and react to reach equilibrium at a
fixed temperature. The equilibrium partial
pressure of NO2 is measured to be 2.4 atm.
Calculate the equilibrium constant of the
reaction at this temperature, assuming that no
competing reactions occur.
21
Initial P (atm)
3.6 5.1 8.0
Change in P (atm)
- x 2x/4 3x/4
Change in P (atm)
- 4x 2x 3x
Equilibrium P (atm)
2.4 5.12x 8.03x
- At equilibrium, the partial pressure of NO2 is
2.4 atm - 3.6 - 4x 2.4 gt x 0.3 atm
- Hence PN2O at equilibrium 5.7 atm PO2 8.9
atm
22
- In applying the equilibrium expression the
following must be considered. - 1) The equilibrium constant for a reverse
reaction is the reciprocal of the equilibrium
constant for the corresponding forward reaction.
23
- 2) When the coefficients in a balanced chemical
equation are multiplied by a constant factor, the
corresponding equilibrium constant is raised to
the power equal to that factor.
K2
PH2 PO2
1/2
24
- 3) When chemical equations are added or
subtracted to obtain a net equation, the
corresponding equilibrium constants are
multiplied or divided to obtain the equilibrium
constant of the net equation.
25
- Adding the two chemical equations gives
Looking at the expressions for K1, K2 and K3 K1
K2 K3 Hence, K3 0.023 at 25oC
26
Reaction Quotient
- For the general reaction
Define the reaction quotient, Q
where P is the partial pressure of a species at
any point in time.
27
- If Q K, the reaction is at equilibrium
- If Q ? K, the reaction is not at equilibrium.
- If Q gt K , reaction proceeds from right to left
- If Q lt K, reaction proceeds from left to right
28
(No Transcript)
29
- The Ideal Gas Equation and Chemical Equilibrium
- For gaseous reactants or products, the
concentration may be in moles/liter. - The concentrations of the gases in moles/liter
must be converted to partial pressures.
Concentration of a species A in moles/lit A
This equation can be used to convert
concentration of a gas in moles/lit to partial
pressure of the gas.
30
Hence, for a general gas phase reaction
- where Pref is the reference pressure 1 atm and
ensures that K is unitless.
31
The equilibrium constant for the reaction CH4(g)
H2O(g) CO(g) 3 H2(g) Equals 0.172 at
900K. The concentrations of H2(g), CO(g), and
H2O(g) in an equilibrium mixture of gases all
equal 0.00642 mol/L. Calculate the concentration
of CH4(g) in the mixture, assuming that this is
the only reaction taking place.
0.00642 mol/L 0.00642 mol/L 3
CH4 0.00642 mol/L
0.172
(0.08206 L atm mol-1) 900 K-2
CH4 0.00839 mol/L
32
- What happens if a system at equilibrium undergoes
a change in conditions? - The tendency of a system to achieve equilibrium
is spontaneous. - Once a system is at equilibrium it will remain at
equilibrium. - However, if conditions change which are different
from the equilibrium condition (for example the
temperature changes) the system will respond to
this change in a way to achieve equilibrium
again. - Note the concentrations of species when
equilibrium is re-established need not be the
same as the ones established at the previous
equilibrium.
33
(No Transcript)
34
- LeChateliers Principle indicates how a system
will respond if a system at equilibrium
experiences a change - If a stress is applied to a system at
equilibrium, the system tends to react so that
the stress is minimized
A) Changing the concentration of a reactant or
product The reaction quotient determines the
direction of the reaction. If the system is at
equilibrium, Q K If to a system at equilibrium
a small amount of reactant is added, Q decreases
and is lt K. Hence the reaction will proceed to
the right to reduce the concentration of the
added reactant.
35
- If a product is removed from the equilibrium
mixture, Q also decreases, and the reaction once
again proceeds to the right to increase the
concentration of the product. - B) Changing the Volume
- Decreasing the volume, increases the total
pressure of the reaction mixture. - The reaction will then proceed in the direction
which reduces the total pressure.
If the volume is decreased, the above reaction
will move to the right, to decrease the total
number of molecules and hence the pressure.
36
- C) Changing Temperature
- The effect of changing the temperature of a
system at equilibrium depends on whether the
reaction proceeds by absorbing energy
(endothermic) or by releasing energy
(exothermic).
Forward reaction is exothermic reverse reaction
is endothermic.
An endothermic reaction lowers the temperature of
the system and an exothermic reaction raises the
temperature of the system. If a reaction is
exothermic, raising the temperature causes the
equilibrium to shift to the left.
37
For reactions where the equilibrium concentration
of a product is low, product yields can be
increased by adjusting conditions that encourage
a reaction to proceed in the direction of the
products.
38
- Heterogeneous Reactions
In all equilibrium expressions, the
concentrations of all pure solids and liquids are
set to 1.
39
- In general, to write the equilibrium expression
for a reaction - 1) Concentration of gases are expressed as
partial pressures - 2) Concentration of dissolved species in solution
are expressed as moles/liter - 3) Concentrations of pure solids and liquids are
set to 1 - (for a solvent taking part in a reaction, its
concentration is also set to 1 providing the
solution is dilute)
40
(No Transcript)
41
42
- Applications of Chemical Equilibria
- Why is CO lethal?
- Hemoglobin (Hb) is the main chemical component of
red blood cells, carrying oxygen from the lungs
to the body tissue, transporting oxygen from a
region of high concentration to low
concentration. - This transportation is accomplished by the
formation of the oxygen-hemoglobin complex,
oxyhemoglobin (HBO2)
43
In the absence of Hb, the amount of O2 in blood
is low. Because of the formation of the HbO2
complex, the amount of O2 in blood is increased
by a factor of 70.
LeChateliers principle predicts that in regions
of high O2 partial pressure, the Hb-HbO2
equilibrium is shifted to the right, which is the
case in the lungs In regions of low O2 partial
pressure, the equilibrium shifts to the left,
resulting in a breakup of the HbO2 complex,
releasing O2 to the bodys tissues.
44
What happens if a person breathes CO?
K(CO) gt (KO2 ) Which means that CO binds more
tightly to Hb compared to O2
45
When Hb is exposed to both O2 and CO, there is
competition for the Hb, and the following
reaction takes place
The K for this reaction is
46
The relative amounts of HbCO and HbO2 therefore
depend on the partial pressures of CO and O2.
Since K(CO) gt K(O2), K for the competition
reaction is gt1 In fact at 38oC, the value of K
is 210 and so the position of the equilibrium
strongly favors the formation of the HbCO
complex, with CO displacing O2 from the HbO2
complex, resulting in asphyxiation. However,
the process is reversible - from LeChateliers
principle, a large partial pressure of O2 will
result in the equilibrium of the competition
reaction to favor the HbO2 complex.
47
- Extraction and Separation
A solute dissolved in a solvent A can be
extracted from this solvent by using another
solvent B. The solute must dissolve in both
solvents A B and solvent B must be immiscible
with the solvent A. For example, CCl4 and H2O
are immiscible. I2(s) dissolves in both solvents.
48
- If to a solution of I2(aq) some CCl4 is added and
then the flask containing the I2(aq) and CCl4 is
shaken, some of the I2 in the water layer will be
extracted into the CCl4 layer
The following equilibrium is then established
49
- Chromatography - extraction of small quantities
of solute