Other Ionic Structures' - PowerPoint PPT Presentation
1 / 6
Title:
Other Ionic Structures'
Description:
For perfect cube: A-O/B-O = 2 'Goldschmidt tolerance factor' ... Many applications: non-volatile memories; sensors, actuators, ultra-sonic imaging ... – PowerPoint PPT presentation
Number of Views:49
Avg rating:3.0/5.0
Title: Other Ionic Structures'
1
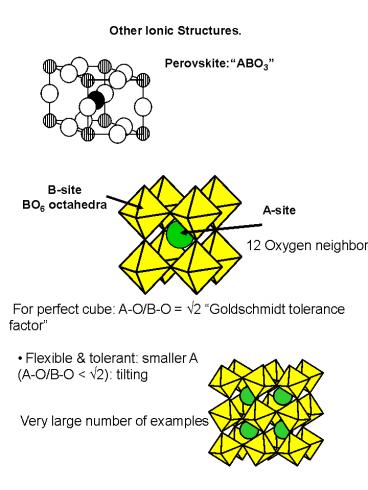
Other Ionic Structures.
PerovskiteABO3
B-site BO6 octahedra
A-site
12 Oxygen neighbors
For perfect cube A-O/B-O v2 Goldschmidt
tolerance factor
- Flexible tolerant smaller A (A-O/B-O lt v2)
tilting
Very large number of examples
2
- Perovskite most widely studied oxide structure
- Wide range of chemistries possible
- - thousands of examples known
Unique properties of perovskites
- high Tc cuprate superconductors- Colossal
Magneto-Resistance (La,SrMnO3)- fast ion
conduction (Li, O2-), batteries, fuel cells-
mixed electronic/ionic conduction, fuel cells-
oxidation/reduction catalysts- ferroelectric /
piezoelectric ceramics (BaTiO3, Pb(ZrTi)O3)-
important mineral structure in lower mantle
(MgSiO3)- frequency filters for wireless
communications Ba(Zn1/3Ta2/3)O3
3
- Dipoles in BaTiO3
- ferroelectric
- piezoelectric
- Many applications non-volatile memories
sensors, actuators, ultra-sonic imaging
- Memories/nano-lithography
Pole sample to align dipoles
Write using nano-tips
4
High Temperature SuperconductorsCuO based
Zero electrical resistance to 135K
5
La3Mn3O3
each substituted Sr oxidizes Mn3 to Mn4
Replace by Sr2
mixed valent Mn show unique electronic/magnetic
properties
6
ZSM-5 silicate based microporous solids
Catalysts Molecular sieves chemical separation