Surface Analysis - PowerPoint PPT Presentation
1 / 29
Title:
Surface Analysis
Description:
laser beam is reflected off a spot on the cantilever to photodiode that detects the ... photodiode then controls force on cantilever so that it remains constant ' ... – PowerPoint PPT presentation
Number of Views:287
Avg rating:3.0/5.0
Title: Surface Analysis
1
Surface Analysis
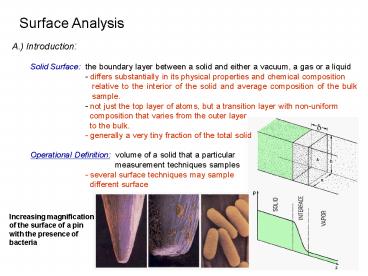
A.) Introduction Solid Surface the boundary
layer between a solid and either a vacuum, a gas
or a liquid - differs substantially in its
physical properties and chemical composition
relative to the interior of the solid and
average composition of the bulk sample. -
not just the top layer of atoms, but a transition
layer with non-uniform composition that
varies from the outer layer to the
bulk. - generally a very tiny fraction of the
total solid Operational Definition volume of
a solid that a particular
measurement techniques samples - several
surface techniques may sample
different surface
Increasing magnification of the surface of a pin
with the presence of bacteria
2
B.) Spectroscopic Surface Methods 1. Provide
chemical information of the composition of a
surface (few D to 10s D thick)
To Spectrometer (secondary beam)
From source (primary beam)
- - Photons, electrons, ions or neutral molecules.
- - Need to limit to surface analysis
- Results from scattering, sputtering or
emission. - Further studied by other
spectroscopic techniques
Sample
Penetration depth of 1-keV electrons is 25 D,
where a photon beam of same energy is 104 D
3
Some Common Types of Spectroscopic Methods for
Analysis of Surfaces
Methods are differentiated by the nature of the
primary and secondary beams
4
2. Sampling Surfaces and Contamination - focus
beam on a single surface - mapping the surface
by scanning the beam across the surface in a
raster pattern can be linear or
two-dimensional observe changes in secondary
beam as a function of position - depth
profiling ? etch a hole in the surface by
sputtering a finer primary beam is used to
generate a secondary beam from the hole
observe changes in secondary beam as a function
of depth
Raster Pattern (data is obtained from primary
beam centered in each grid)
Depth Profiling
5
- contamination of the surface from the
atmosphere or the primary beam - need to clean
surface in vacuum chamber bake sample at
high temperatures sputtering the sample
with a beam of inert gas ions from electron
gun mechanical scraping or polishing the
surface with an abrasive ultrasonic washing
in various solvents bathing the sample in a
reducing atmosphere to remove oxides
Surface contamination
6
3. Electron Spectroscopy - signal from sample
is a beam of electrons (secondary beam) -
determine the power of the beam as a function of
the energy of the electrons (hn) -
determine identification of all elements
(except H and He) oxidation state of
element type of species element is bonded
to electronic structure of molecules -
poor penetration power of electrons
restricted to surface layers of 20 50
D -qualitative analysis of the surfaces of
metals, alloys, semiconductors and
heterogeneous catalysts
Different element abundance in different regions
of the surface
7
A). Electron Photoelectron Spectroscopy (XPS) i.
Theory - record kinetic energy of emitted
electron plot of number of emitted electrons
as a function of the energy of the emitted
electron - X-ray beam of known energy (hn)
displaces an electron A hn ? A
e- -Kinetic energy of emitted electron (Ek) is
measured in an electron spectrometer Binding
energy (Eb) of the electron is Eb hn - Ek
- w where hn energy of electron
beam Ek measured kinetic energy of emitted
electron w work function of spectrometer
(determined experimentally)
Photoelectric Process
8
- well separated peaks for each element on the
surface can be observed except hydrogen -
observe multiple peaks for each element S, P
and D orbital energy decreases as a function
of orbital S gt P gt D energy increases as
a function of atomic number N1s gt C1S
Binding Energy of an electron is characteristic
of the atom and the orbital from which the
electron was emitted
Binding Energy (eV)
9
ii. Instrumentation - Expensive (300,000 -
900,000) - Requires extensive vacuum systems
(10-5 to 10-10 torr) - Similar to optical
spectrometers
10
- X-ray source (X-ray tube) bombardment of
a metal target with a beam of high-energy
electrons - electrons produced at heated
cathode - accelerated to metal anode, copper
block with target (Mg, Al) - large potential
(100 kV) X-rays produced by decelerated
electrons upon collision with target - energy
of photon is equal to the difference in kinetic
energy of the electron before and after
collision. - deceleration occurs as a series
of collisions ?different kinetic energy
losses - X-ray energies vary over a
continuum Very inefficient, less than 1 of
electrical power converted to X-rays -
remainder dissipated as heat - water cooling
is required
11
- Sample holders samples mounted as close
to X-ray source as possible - avoid
attenuation of X-ray sample container under
a vacuum (lt10-5 torr, typically 10-9 to 10-10
torr) - avoid contamination of sample surface
by water or oxygen - avoid attenuation of
X-ray beam due to inelastic collisions -
Analyzers hemispherical type electron
beam deflected by an electrostatic magnet -
electrons travel in a curved path - radius of
curvature is dependent on kinetic energy of
electron - vary field, direct electrons of
different kinetic energy onto detector.
under a vacuum (lt10-5 torr) - Transducer
(detector) solid-state, channel electron
multipliers tubes of glass doped with lead or
vanadium - apply a potential of several
kilovolts across these materials - a cascade
of electrons (106 -108) for each incident
electron - pulses are counted
electronically similar in concept to
photomultiplier tubes
12
iii. Chemical Shifts and Oxidation States -
position of a peak is dependent on the chemical
environment of the atom - variations in the
number of valence electrons and type of bond
influence the binding energy of the core
electrons
Binding energy increases as a function of
electronegativity Similar effects observed for
different Oxidation states
13
Similar effects observed for different Oxidation
states
Electron volts relative to oxidation state marked
by
14
B). Auger Electron Spectroscopy (AES) i.
Theory - two-step process, generates Auger
electron differs from XPS 1) generate
excited ion as before A hn ? A
e- or using an electron beam A
ei- ? A ei- eA- where
ei- is the beam electron after interaction
with A and loss of kinetic
energy. eA- is ejected from A inner
orbital 2) excited ion may relax by emitting
an Auger electron (eA-) with kinetic
energy (Ek) A ? A eA- or by
fluorescence (X-ray fluorescence) A ? A
hnf -Kinetic energy of emitted electron (Ek) is
independent of the energy of photon or
electron that Kinetic energy (EK) of the
Auger electron is EK (Eb Eb) Eb Eb
2Eb where (Eb-Eb) energy
released in relaxation of the excited ion
Eb energy required to remove the second
electron from its orbit
15
- Auger emissions are described in terms of the
type of orbital transitions involved in the
production of an electron KLL 1.
removes a K electron 2. transition of an L
electron to the K orbital 3. ejection of a
second L electron LMM and MNN are also
common transition - measurement of the kinetic
energies of the emitted electrons each
element in a sample being studied will give rise
to a characteristic spectrum of peaks
at various kinetic energies. Spectra
typically displayed as a derivative (dN(E)/dE)
- enhance small peaks, repress effects of
intense peaks - Auger electron emission and
X-ray fluorescence are competitive processes
lower atomic number ? Auger electron emission
higher atomic number ? X-ray fluorescence
AES Sample Pd Ep 2.5 Kev
16
ii. Advantages - sensitivity to atoms of low
atomic number - minimal matrix effects - high
spatial resolution detailed examination of
solid surfaces electron beam more tightly
focused than X-ray beam iii. Disadvantages -
not used to provide structural and oxidative
state information (XPS) - quantitative analysis
is difficult iv. Instrumentation - similar to
XPS, most instruments do both XPS and AES -
requires an electron gun
17
Diagram of Electron Gun
- Heated tungsten filament
- 0.1 mm diameter
- Bent into shape of hairpin with V-
- shaped tip
- maintained at potential of 1 -50 kV
- Wehnelt cylinder
- surrounds filament
- negative bias to filament (0-3000V)
- electric field of Gun causes electrons to
- converge at crossover
- Produces a beam of electrons of 1 to 10 keV
- can be focused onto the surface with diameters
of 500 to 5 mm
18
4. Scanning Electron Microscopy - high
resolution images of surfaces optical
microscopy limited by wavelength of light,
diffraction effects - surface is swept
in raster pattern with finely focused beam of
electrons swept in straight line in
x-direction returned to starting point
shifted downward (y direction) by a standard
direction
Cecropia Moth Scales (15,000x)
Cecropia Moth Scales (350x)
19
A). Scanning Electron Microscope (SEM) i.
Introduction - surface is scanned with beam of
electrons - several types of signals from the
surface are created backscattered or
secondary electrons - two most common for SEM
X-ray fluorescence Auger electrons
- -Condensing lens and objective lens reduce
electron beam to final spot of 5 to 200 nm - Two magnet coils in objective lens deflect e-
beam in x and y direction to scan surface - intensity of beam created from surface are used
to drive the horizontal and vertical scans of a
cathode-ray tube (CRT). - Magnification (M) W/w
- where
- W width of CRT
- w width of single line
- scan across the sample
20
- increased magnification is achieved by
decreasing w narrower the beam of electrons
the higher the magnification magnification
range is 10x to 100,000x
Increasing Magnification of Mosquito
- samples changers are designed for rapid
changing of samples large-capacity vacuum
pumps bring pressure to lt 10-4 torr can
hold samples many centimeters in length can
be moved in x, y and z directions - sample
considerations samples that can conduct
electricity are easiest to study - flow of
electrons to ground minimize artifacts - also
typically good conductors of heat - minimize
thermal degradation most biological and
mineral samples do not conduct electricity -
coat surface with thin metallic film by
sputtering or vacuum evaporation -
detectors scintillation device -
doped glass or plastic emits photons when struck
by electrons
21
- detectors scintillation device -
doped glass or plastic emits photons when struck
by electrons - photons conducted by light
tube to photomultiplier tube (outside
instrument) - gains in signal of 105 to 106
ii. Interaction of Electron Beams with
Surface - elastic interactions affect the
trajectory of electron not energy deflection
of collision is random (0 to 180 deg.) beam
penetrates to a depth of 1.5 mm or more
eventually exit from surface as backscattered
electrons - larger diameter? limits
resolution - inelastic interactions
transfer part or all of energy from electron to
solid excited solid emits secondary
electrons, Auger electrons, X-rays
Simulation of Electron Trajectories
22
- Secondary Electron Production electrons
having energies of 50 keV or less are emitted
from surface - along with backscattered
electrons - ½ to 1/5 number of backscattered
electrons beam ejects conduction band
electrons only from depth of 50 to 500 D
slightly larger beam than incident beam
prevent from reaching transducer by a small
negative bias - X-ray emission
characteristic line spectra and continuum are
produced
- (1) column hosts the electron beam.
- (2) microscope is operated from the steering
panel - (3) cryo-unit with a binocular
- exchange chamber is used to introduce the object
into the high vacuum area. - The object can be observed on the large screen
while it is scanned. - The small screen serves to watch the object
chamber. - The computer for image archiving and processing.
- electronics
23
5. Scanning Probe Microscopes - resolve
details of surface to the atomic level Nobel
Prize in 1986 fro G. Binnig and H. Roher -
provides details in x, y and z dimension 20
D resolution in x, y as low as 1 D
resolution in z better than 1 D electron
microscope resolution is 50 D
direct observation of standing-wave patterns in
the local density of states of the Cu(111)
surface. These spatial oscillations are
quantum-mechanical interference patterns caused
by scattering of the two-dimensional electron gas
off the Fe atoms and point defects.
24
A). Scanning Tunneling Microscope i.
Introduction - surface must conduct
electricity - surface is scanned in raster
pattern with a very fine metallic tip - tip is
maintained at constant distance from sample by
maintaining a constant tunneling current
up and down motion of tip reflects topology
of surface tunneling current created by
voltage between tip and sample
Constant (d)
V
Scanned graphite surface
STM tip scanning graphite surface (spheres are
carbon atoms)
25
ii. Tunneling current - current that passes
through a medium that contains no electrons
vacuum, nonpolar liquid, aqueous electrolyte
solution explained by quantum mechanics -
tunneling currents become significant when
two conductors are within a few nanometers of
each other one conductor is in the form of a
sharp tip - tunneling current (It) is given
by It Ve-Cd where V voltage between
conductors C constant dependent on
composition of conductors d spacing between
tip and surface - tunneling current is held by
moving tip up and down so d is constant tip
is moved by potential change in x, y or z
dimension that causes a hollow-tude
piezoelectric device to bend in the x,y plane or
extend/shrink along z-axis
Image of STM Tip
The inner and outer curved surfaces are coated
with a thin metal layer to form two electrodes.
The piezo tube bends under application of unequal
voltages to the electrodes. Accuracy of movement
is on the order of 1 nm
26
iii. Tips - critical to performance of SEM -
best images when the end of the tip is a single
metal atom tunneling current increases by 10x
when gap distance decreases by 1 D all
current flows to single atom that is 1 D closer
to surface than all other atoms - tips
constructed by cutting platinum/iridium
wires electrochemical etching of tungsten
metal exponential increase in tunneling
current with decreasing gap makes it
possible to prepare single atom tip
Image of STM Tip
SEM used to move Fe atoms
27
B). Atomic Force Microscope (AFM) i.
Introduction - resolution of individual atoms
on both conducting and insulating surfaces
STM requires conducting surface - flexible
force-sensing cantilever stylus is scanned over
the surface - force acting between cantilever
and surface causes minute deflections of the
cantilever deflections detected by optical
means motion of cantilever controlled by
piezoelectric tube (same as STM)
1. Laser 2. Mirror 3. Photodetector 4.
Amplifier 5. Register 6. Sample 7. Probe 8.
Cantilever
Bending or tapping of cantilever as it moves
across service
Images of cantilever and tip
28
- laser beam is reflected off a spot on the
cantilever to photodiode that detects the
motion output from phtodiode then controls
force on cantilever - output from photodiode
then controls force on cantilever so that it
remains constant similar to tunneling current
control in STM motion of cantilever
controlled by piezoelectric tube (same as STM)
ii. Tips and Cantilever - critical to
performance of AFM - originally crushed
diamonds attached to metal foil - currently,
tips constructed by etching single chips of
silicon, silicon oxide or silicon nitride
remarkably small and delicate - few tens of mm
in length, less then 10 mm in width, 1 mm
thickness - tips are few mm in height and
width
Can view cantilever as coil spring
29
iii. Tapping Mode Operation - disadvantages of
contact mode scanning potential damage of
surface and distortion of image especially
problematic with soft samples - biological
samples - polymers - silicon wafers -
Tapping mode only allow tip to contact
surface briefly then removed from surface
cantilever oscillates at frequency of few 100
kHz image material that was impossible to
image by contact mode
Atomic force microscopy images of reconstituted
nucleosomal arrays. (A) A chromatin fiber with a
beads-on-a-string morphology reconstituted at 1
1 histone octamers to DNA ratio. (B) When 1.5 1
histone octamers to DNA ratio is used for
reconstitution a condensation of the core histone
octamers is observed