Chemical manufacturing processes - PowerPoint PPT Presentation
1 / 18
Title:
Chemical manufacturing processes
Description:
Water/acetone. Water/chloroform. Aqueous two-phase systems. Sodium phosphate/PEG in water ... A, Acetone. C, MIBK. Water, B. J. F. K. H. L. P. Q. More partially ... – PowerPoint PPT presentation
Number of Views:707
Avg rating:3.0/5.0
Title: Chemical manufacturing processes
1
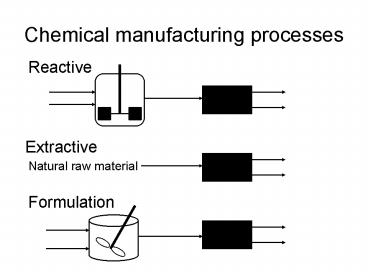
Chemical manufacturing processes
Reactive
Extractive
Natural raw material
Formulation
2
Basis of separation
- Size
- Shape
- Charge
- Polarity
- Solubility
- Volatility
- Mobility
3
Classification of separation processes
- Based on position of equilibrium
- Equilibrium
- Non-equilibrium
- Based on nature of process streams
- Phase transfer
- Stream splitting
4
Separation processes
- Liquid-liquid extraction
- Adsorption
- Filtration
- Solid-liquid extraction (leaching)
- Elution chromatography
- Membrane separation processes
- Distillation
- Affinity separation processes
- Drying and evaporation
- Freeze-drying
- Precipitation
- Crystallization
- Electrophoresis
- Centrifugation
- Mechanical sieving
5
Staged separations Phase transfer
Cross-current
Co-current
Rectification type
Counter-current
6
Staged separations Stream splitting
7
Liquid-liquid extraction
- Transfer of solute from on liquid to another
- Depends on the partition behavior of the solute
- Used when
- Solute and solvent have similar volatility
- Solute is thermolabile
- Solute and solvent form azeotropic mixture
- Solute concentration is low
8
Applications of Liquid-liquid extraction
- Separation of aromatics from hydrocarbons
- Purification of antibiotics
- Purification of aromatics such as benzene, xylene
and toluene - Protein purification using aqueous two-phase
systems - Purification of natural products
- Purification of dyes and pigments
- Metallurgical purifications
9
Extractors
10
Solvent systems
- Aqueous / non-aqueous systems
- Water/MIBK
- Water/acetone
- Water/chloroform
- Aqueous two-phase systems
- Sodium phosphate/PEG in water
11
Solvent miscibility
- Completely miscible
- Unsuitable for extraction
- Immiscible
- Ideally suited for extraction
- Partially miscible
- Composition dependent
- Various possibilities
- Could be used for extraction
12
Solute portioning
13
Partially miscible solvent system
A, Acetone
F
K
J
P
H
Q
C, MIBK
Water, B
L
14
More partially miscible solvent systems
A
A
C
B
C
B
15
Liquid-liquid extraction Sequence of events
- Mixing or contacting
- Phase separation
- Collection of separate phases
16
Liquid-liquid contacting
- Shaking the extraction device
- Using an stirred tank
- Counter-current liquid flow
- Agitated counter-current liquid flow
- Contacting across a porous structure
- Ultrasonic vibration
17
Separation of phases
- Coalescence and phase separation due to density
difference - Coalescence and phase separation by centrifugal
assistance - Assisted coagulation of dispersed phase
- Separation by membrane
18
Solute mass transport
- Rate depends on the specific interfacial area and
mass transfer coefficient - Rate k a ?C
- Both of these depend on system hydrodynamics