Gases - PowerPoint PPT Presentation
1 / 22
Title:
Gases
Description:
Gas Density and Molar Mass ... Gas Mixtures and Partial Pressures ... However, the gas is saturated with water vapor ... – PowerPoint PPT presentation
Number of Views:185
Avg rating:3.0/5.0
Title: Gases
1
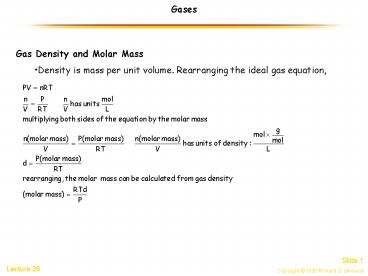
Gases
- Gas Density and Molar Mass
- Density is mass per unit volume. Rearranging the
ideal gas equation,
2
Gases
- Gas Density and Molar Mass
- Example In the Dumas-bulb technique for
determining the molar mass of an unknown liquid,
you vaporize the sample of a liquid that boils
below 100 oC in a boiling-water bath and
determine the mass of vapor required to fill the
bulb. From the following data, calculate the
molar mass of the unknown liquid mass of
unknown vapor, 1.012 g volume of bulb, 354 cm3
pressure, 742 torr temperature, 99 oC.
3
Gases
- Stoichiometry of Reactions Involving Gases
- Avogadros Hypothesis states that equal volumes
of gases at the same temperature and pressure
contain the same number of molecules. - Application to stoichiometry the coefficients in
balanced equations can represent volumes of
gaseous substances as well as moles or molecules. - Example N2(g) 3H2(g) 2NH3(g)
- At a given temperature and pressure, 1 L of N2(g)
reacts with 3 L of H2(g) to produce 2 L NH3(g). - Stoichiometric volume equivalences or ratios can
be written - 1 L N2(g) Û 3 L H2(g)
- 3 L H2(g) Û 2 L NH3(g) etc.
4
Gases
- Example What volume of NH3(g) is produced at
1.00 atm and 0.00 oC from 10.0 L N2(g) at 150
oC and 800 torr and 18.0 L H2(g) at 200 oC and
350 torr? - One way to solve this problem is to convert the V
of N2(g) and H2(g) to a common T and P well
use the T and P at which the product NH3(g) is
formed.
Another way to solve this problem would be to
convert the volumes to moles, calculate the of
moles NH3 produced and convert to L NH3 at 1.00
atm and 0.00 oC.
5
Gases
- Example Calculate the volume of gas produced in
a chemical reaction - What volume of H2 at 25 oC and 610 torr is
produced from 3.00 g Zn? - Zn 2HCl ZnCl2(aq) H2(g)
6
Gases
- Gas Mixtures and Partial Pressures
- The partial pressure of a gas is the pressure
that would be exerted by a single gas in a
mixture of gases in the absence of the other
gases. - Daltons Law of Partial Pressures states that the
total pressure exerted by a mixture of gases is
the sum of the partial pressures of each gas in
the mixture. - PtotalP1 P2 P3
- This equation comes about because each ideal gas
in a mixture behaves independently - each kind
of molecule behaves like any of the other
molecules. - If each gas in the mixture behaves like an ideal
gas
- In a mixture, all the gases are at the same T and
are contained in the same V
7
Gases
- Gas Mixtures and Partial Pressures
- Example What is the total pressure exerted by a
mixture of 2.00 g H2 and 8.00 g of N2 at 273 K
in a 10.0 L container?
8
Gases
- Partial Pressures and Mole Fractions
- The ratio of the partial pressure of a gas to the
total pressure of a mixture gives the ratio of
the moles of a gas to the total moles of gas in
the mixture.
- X1 is the mole fraction of gas 1 in the mixture
- The partial pressure of a gas is the mole
fraction times the total pressure. - Example Data from Voyager 7 give information
about the composition of the atmosphere of
Titan, Saturns largest moon. The total pressure
is 1220 torr and the atmosphere consists of 82
mol percent N2, 12 mol percent Ar and 6.0 mol
percent CH4. What is the partial pressure of
each gas?
9
Gases
- Collecting Gases Over Water a common laboratory
method is to produce a gas by some chemical
reaction and displacing water from a container. - This allows the gas to be collected without
mixing with air - The volume of the water displaced allows
determination of the volume of the gas produced
in the reaction. - However, the gas is saturated with water vapor
- If the gas is collected in such a way the
pressure of the gas/water vapor mixture is the
atmospheric pressure - Patm Pgas Pwater
- Pgas Patm - Pwater
- Pwater is the vapor pressure of water at the
temperature of the water and is tabulated in
your text in Appendix G.
10
Gases
- Collecting Gases Over Water
- Example NH4NO2 can be decomposed to produce
N2(g). A sample of NH4NO2 was decomposed and 511
mL N2 collected over water at 26 oC and 745 atm.
How many grams of NH4NO2 were decomposed? - NH4NO2(s) N2(g) 2H2O(g)
11
- Gases
- Kinetic-Molecular Theory of Gases explains why
gases follow the gas laws. - Postulates of the theory
- Gases consist of a large number of molecules that
are in constant, random motion. - The volume of gas molecules is negligible
compared to the total volume of their container. - Attractive and repulsive forces between gas
molecules are negligible - Collisions between gas molecules are perfectly
elastic energy can be exchanged between gas
molecules by collision, but the average energy of
the molecules in a gas is constant at constant
temperature and does not change with time. - The average kinetic energy of gas molecules is
proportional to the absolute temperature and
independent of the kind of gaseous molecules. - Distribution of molecular speeds see Fig 12.18,
p. 566. - Not all the molecules have the same velocity -
there is a distribution in the speeds of the
molecules in a gas at constant temperature. - If the temperature increases the distribution
moves to higher velocities. - The root-mean-square velocity is the velocity of
a molecule having the average kinetic energy.
12
(No Transcript)
13
Gases
- Kinetic Molecular Theory of Gases
- The rms speed is the square root of the sum of
the squares of the speeds of the molecules in a
sample of gas divided by the number of molecules
in the sample. - The average kinetic energy is calculated from the
rms speed - e mu2 (see Fig. 12.19, p. 567, for the
effect of molecular mass on distribution of
molecular velocity) - m is constant for a given gas, so if energy is
added to a gas, u must increase. - Pressure is caused by molecular collisions with
the walls of the container - The faster the molecules move the greater the
force of collision with the walls of a
container. - The faster the molecules move the more often the
molecules will collide with the walls of a
container. - Temperature is a measure of the average kinetic
energy of gas molecules. - The higher the temperature the faster the
molecules move
14
(No Transcript)
15
Gases
- Kinetic Molecular Theory of Gases
- Explanation of Boyles Law - Pressure - Volume
Relationship - At constant temperature the gas molecules have a
particular rms velocity. - If the volume of the container increases, the
distance molecules move before collision with
the walls increases. - The number of collisions per unit time decreases
so the pressure drops if volume increases. - Explanation of Gay-Lussacs Law - Temperature -
Pressure Relationship - At constant volume and temperature, the number of
collisions per unit time with the walls of the
container is constant so the pressure is
constant. - If the temperature increases at constant volume,
the kinetic energy, and thus the rms velocity of
the gas molecules increases. - The number of collisions with the container walls
per unit time increases and the force of the
collisions increases so the pressure increases
with increasing temperature.
16
Gases
- Kinetic Molecular Theory of Gases
- Explanation of Charless Law - Volume -
Temperature Relationship - Increasing the temperature of a gas at a given
pressure increases the average kinetic energy of
the gas molecules and thus their rms speed. - This increases the number of collisions with the
container walls per unit time and increases the
force of the collisions. - In order to maintain constant pressure the volume
must increase to increase the distance
molecules travel between collisions and reduce
the number of collisions with the container walls
per unit time. - Thus, volume increases with temperature at
constant pressure. - Explanation of Avogadros Law Volume - Amount of
Substance Law - Increasing the number of molecules in a container
at constant temperature will increase the number
of collisions per unit time with the container
walls. - To maintain constant pressure, the volume of the
container must increase in order to make the
number of collisions with the wall constant.
17
Gases
- Kinetic Molecular Properties of Gases
- Because the average kinetic energy of gas
molecules mu2 - two different gases with different m values at
the same T, thus same e - e1 m1u12 m2u22 e2
- since the molar mass is proportional to the
molecular mass - and it can be shown
- For a given temperature, the higher the molar
mass, the smaller the rms velocity. - See Fig. 12.9, p. 567, showing velocity
distributions for molecules with different
molar masses all at 25 oC.
18
Gases
- Kinetic Molecular Properties of Gases
- Grahams Law of Effusion Effusion is the passage
of gas molecules through a small hole from a
high pressure region into a vacuum. - The higher the rms speed of molecules the higher
the rate of effusion
- Example H2 effuses 2.9 times as fast as unknown
gas at the same temperature. What is the molar
mass of the unknown?
19
Gases
- Kinetic Molecular Properties of Gases
- Diffusion and Mean Free Path
- Diffusion is the process by which molecules move
from an area of high concentration to an area
of lower concentration. - The rates of diffusion can be approximated by
Grahams Law - Diffusion is not instantaneous even though rms
speeds are hundreds of meters/second because
gas molecules under normal conditions undergo
many collisions per second - perhaps 1010 per
second at 1 atm and 25 oC. - The average distance molecules travel between
collisions under normal conditions is 10s of
nm. This is the mean free path. - Real Gases Deviations from Ideal Behavior
- should equal 1 for 1 mole of ideal
gas at all pressures and temperatures. - Generally deviations are significant only at
pressures much greater than 1 atm.
20
Gases
- Real Gases Deviations from Ideal Behavior
- At high pressures, the gas molecules are close
enough that their volume is not a small fraction
of the volume occupied by the bulk sample. - At high pressures, the gas molecules are close
enough that attractive forces between gas
molecules become important. - The attraction between molecules at the walls of
a container with nearby molecules reduces the
force of impact of molecules with the wall and
thus reduce the expected pressure. - This reduces from its expected value of 1
- At very high pressures, the molecular volume
increases above its expected value
because gas molecules are not very compressible. - At low temperatures, the gas molecules do not
have as much kinetic energy as they have at high
temperatures. - Its harder for low temperature molecules to
overcome attractive forces and - is less than its expected value at low P.
- At high temperatures and pressures, is
greater than its ideal value because the volume
of the gas molecules becomes important.
21
Gases
- Real Gases Deviations from Ideal Behavior
- van der Waals Equation of State
- the ideal gas law
- Correct this equation for the effect of the
molecular volume and the attraction between
molecules
The volume in which the molecules are free to
move is reduced from the bulk volume by the
volume per mole of gas molecules (approximately
b) times n The pressure is reduced due to
attractions between molecules at high pressure.
For pairs of interacting molecules the effect
goes like the square of the molar density. The
value of a indicates how strongly molecules of
a gas are attracted to one another. Values for
the van der Waals constants a and b are given in
Table 12.3, p 572.
22
- Gases
- Real Gases Deviations from Ideal Behavior
- van der Waals Equation of State
- Example 1.000 mol of ideal gas at 22.41 L and
0.00 oC would exert a pressure of 1.000 atm. Use
the van der Waals equation to calculate the
pressure of 1.000 mol of Cl2 gas at 22.41 L and
0.00 oC. - From Table 12.3, a6.49 L2-atm/mol2 and b0.0562
L/mol
The 1.003 atm includes a correction to pressure
due to molecular volume. The 0.013 atm includes
a correction to pressure due to molecular
attraction. The molecular attraction between Cl2
molecules at 1 atm and 0.0 oC is the main
reason this gas deviates from ideality.