Chapter 18: Equilibria in Solutions of Weak Acids and Bases
1 / 39
Title:
Chapter 18: Equilibria in Solutions of Weak Acids and Bases
Description:
If the solute concentration is too small, or the equilibrium constant too large, ... For buffer solutions, the initial concentration of both the weak acid and its ... –
Number of Views:171
Avg rating:3.0/5.0
Title: Chapter 18: Equilibria in Solutions of Weak Acids and Bases
1
Chapter 18 Equilibria in Solutions of Weak Acids
and Bases
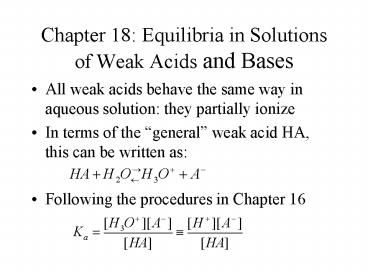
- All weak acids behave the same way in aqueous
solution they partially ionize - In terms of the general weak acid HA, this can
be written as - Following the procedures in Chapter 16
2
- Ka is called the acid ionization constant
- These are often reported as the pKa
- Table 18.1 and Appendix C list the Ka and pKa for
a number of acids - A large pKa,means a small value of Ka and
only a small fraction of the acid molecules
ionize - A small pKa,means a large value of Ka and a
large fraction of the acid molecules ionize
3
- Weak bases behave in a similar manner in water
- For the general base B
4
- Values of Kb and pKb for a number of weak bases
are listed in Table 18.2 and in Appendix C - Where, like for acids
- There is an interesting relations ship between
the acid and base ionizations constants for a
conjugate acid-base pair - Using the general weak acid HA
5
6
- Thus, for any conjugate acid-base pair
- Most tables of ionization constants only give
values for the molecular member of the conjugate
acid-base pair - The ionization constant of the ion member of the
conjugate acid-base pair is then calculated as
needed
7
Relative strengths of conjugate acid-base pairs.
The stronger the acid is, the weaker the
conjugate base. The weaker the acid, the stronger
the conjugate base. Very strong acids ionize 100
and their conjugate bases do not react to any
measurable extent.
8
- The primary goal is usually to determine the
equilibrium concentration for all species in the
mass action expression - The percentage ionization of the acid or base is
defined as - This, and the pH, are often used or requested in
equilibrium calculations
9
- Example Morphine is very effective at relieving
intense pain and is a weak base. What is the Kb,
pKb, and percentage ionization of morphine if a
0.010 M solution has a pH of 10.10? - ANALYSIS The reaction can be represented as
- At equilibrium, OH- x 10-pOH
10
- SOLUTION Use pOH 14.00 pH, substituting
11
Determining how to proceed in acid-base
equilibrium problems. The nature of the solute
species determines how the problem is approached.
This flowchart can help get you started in the
right direction.
12
- As in Chapter 16, if the extent of reaction (x)
is small it is possible to simplify the mass
action expression - For the case of a weak acid or base added to pure
water - If the initial solute concentration is at least
400 times larger than the ionization constant,
the initial concentration of the solute can be
used as though they were the equilibrium
concentration - If the solute concentration is too small, or the
equilibrium constant too large, then the
quadratic equation must be used
13
- Ions can also be acids or bases
- For example, NH4 is a weak acid and NO2- a weak
base - In salts both anions and cations are present,
either of which could affect the pH - These ions can arise from a number of sources
- Properties of their aqueous solutions can be
summarized
14
- Aqueous cations
- Cations that are conjugate acids of weak
molecular bases are weak acids - Metal cations with a high charge density (like
Al3, Fe3, and Cr3) yield aqueous solutions
that are acidic - Aqueous anions
- The anion of a strong acid is too weak a base to
influence the pH of a solution - Anions of weak acids tend to make solution basic
15
- There are four possibilities when a salt is added
to pure water - Neither the anion nor cation affects the pH and
the solution remains neutral. For example NaCl - Only the cation is acidic, so the solution
becomes acidic. For example NH4Cl - Only the anion is basic, so the solution becomes
basic. For example NaNO2 - The anion is basic and the cation is acidic, the
pH of the solution will be determined by the
relative strengths of the acid and base. For
example NH4NO2 produces an acidic solution and
NH4OCl produces a basic solution
16
- The quadratic equation can be used to solve
equilibrium problems when simplifying assumptions
are invalid - Simplifying assumptions fail, for example, when
the initial concentration of a weak acid or base
in pure water is less that 400 times the
ionization constant - Solving the problem using the quadratic equation
is more time consuming, so it is worth checking
before it is used
17
- Example Calculate the pH of a 0.0010 M solution
of dimethylamine for which Kb9.6x10-4. - ANALYSIS 400 Kbgt0.0010 M, so use of the
quadratic equation is indicated. - SOLUTION Set the problem up
18
- Put in standard form
- Solve for x and the equilibrium concentrations
19
- When a small amount of strong acid or base is
added to certain solutions, only a small change
in pH is observed - These solutions are called buffers
- Buffer solution usually contains two solutes, one
providing a weak acid and the other a weak base - If the weak acid is molecular, then the conjugate
base can be supplied as a soluble salt of the acid
20
- Buffers work because the weak acid can react
with added base and the weak base can react with
added acid - Consider the general buffer made so that both HA
and A- are present in solution - When base (OH-) is added
- When acid (H) is added
- Net result small changes in pH
21
- Because of these reactions, calculations
involving buffer solutions can be greatly
simplified - For buffer solutions, the initial concentration
of both the weak acid and its conjugate base can
be used as though they were equilibrium values
(more complicated buffer systems where this
assumption is not valid will not be encountered
in this text) - For buffer solutions only, either molar
concentrations or moles can be used in the Ka (or
Kb) expression to express the amounts of the
members of the conjugate acid-base pair (the same
units must be used for both members of the pair)
22
- Example What is the pH of a buffer made by
adding 0.10 mol NH3 and 0.11 mol NH4Cl to 2.0 L
of solution? The Kb for ammonia is 1.8x10-5 - ANALYSIS This is a buffer, initial
concentrations can be used as equilibrium values
23
- SOLUTION Solve for OH- and use this to
calculate the pH - There are two important factors that determine
the pH of a buffer solution
24
- For the general weak acid HA
- Thus both the value of Ka and the ratio of the
molarities (or the ratio of moles) affect the pH - These last two relations are often expressed in
logarithmic form
25
- The first is called the Henderson-Hasselbalch
equation, and is frequently encountered in
biology courses - When preparing a buffer, the concentration ratio
is usually near 1, so the pH is mostly determined
by the pKa of the acid
26
- Typically, the weak acid is selected so the the
desired pH is within one unit of the pKa - A buffers capacity is determined by the
magnitudes of the molarities of its components - Generally, the pH change in an experiment must be
limited to about
27
- Example A buffer made from 0.10 mol HA
(pKa7.20) and 0.15 mol NaA in 2.0 L has 0.02 mol
of HCl added to it with no volume change. What is
the pH change? - ANALYSIS This buffer problem is best solved in
terms of moles. HCl is a strong acid. The H it
contributes to solution increases the amount of
HA present at the expense of A-. - SOLUTION The pH before addition of HCl was
28
- After the HCl ionizes and reacts
- The pH change was greater than 0.1 unit. The
buffer effectively resisted the pH change,
however, because if the HCl had been added to
pure water, the pH change would have been much
larger
29
- Acids that can donate more than one H to
solution are called polyprotic acids - Table 18.3 (and Appendix C) list the ionization
constants of a number of polyprotic acids - The ionization constants for these acids are
numbered to keep tract of the degree of
ionization - Note that, for a given polyprotic acid, the
magnitudes of the ionization constants are
always Ka1 gt Ka2 (gt Ka3, if applicable)
30
- Simplifying assumptions like those made for the
acid ionization can be made - The overall procedure for an acid-base titration
was discussed in Section 5.13 - The titration stops at the end point, as
indicated by a color change of an acid-base
indicator
31
- If the indicator was properly selected, the end
point is very near the equivalence point (when
stoichiometric amounts of acid and bases have
been added) for the system - If the pH of the solution is plotted versus
volume of titrant added, a titration curve is
obtained - The pH of the solution can easily be obtained
using a pH meter - Some of the more important types of titrations
will be considered
32
- Titration of a strong acid by a strong base
Titration curve for the titration of 25.00 mL of
0.2000 M HCl (a strong acid) with the 0.2000 M
NaOH (a strong base). The equivalence point
occurs at 25.00 mL added base with a pH of 7.0
(data from Table 18.4).
33
- Titration of a weak acid by a strong base
- This can be divided into four regions
- Before the titration begins this is simple a
solution of weak acid - During the titration, but before the equivalence
point the solution is a buffer - At the equivalence point the solution contains a
salt of the weak acid, and hydrolysis can occur - Past the equivalence point the excess added OH-
is used to determine the pH of the solution - Data for the titration of acetic acid with sodium
hydroxide is tabulated in Table 18.5
34
The titration curve for the titration of 25.00 mL
of 0.200 M acetic acid with 0.200 M sodium
hydroxide. Due to hydrolysis, the pH at the
equivalence point higher than 7.00 (data from
Table 18.5).
35
- Titration of a weak base by a strong acid
- This is similar to the titration of a weak acid
by strong base - Again dividing into four regions
- Before the titration begins this is a solution
of a weak base in water - During the titration, but before the equivalence
point the solution is a buffer - At the equivalence point the solution contains
the salt of the weak base, and hydrolysis can
occur - Past the equivalence point excess added H
determines the pH of the solution
36
Titration curve for the titration of 25.00 mL of
0.200 M NH3 with 0.200 M HCl. The pH at the
equivalence point is below 7.00 because of the
hydrolysis of NH4.
37
- Titration curves for diprotic acids
- The features are similar to those for monoprotic
acids, but two equivalence points are reached
The titration of the diprotic acid H2A by a
strong base. As each equivalence point is
reached, the pH rises sharply.
38
- A few general comments about indicators can be
made - Most dyes that are acid-base indicators are weak
acids, which can be represented as HIn - The color change can be represented as
39
- The color change will appear to the human eye
near the equivalence point of the indicator - At the equivalence point, the concentration of
the acid and base form are equal, so that - The best indicators have intense color(s) so only
a small amount will produce an intense color
change that is easy to see and wont consume
too much of the titrant