Event Reduction in Angiographic Plaque Regression Trials PowerPoint PPT Presentation
1 / 26
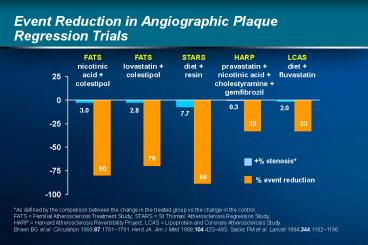
Title: Event Reduction in Angiographic Plaque Regression Trials
1
Event Reduction in Angiographic Plaque Regression
Trials
FATS nicotinic acid colestipol
FATS lovastatin colestipol
STARS diet resin
HARP pravastatin nicotinic acid
cholestyramine gemfibrozil
LCAS diet fluvastatin
stenosis event reduction
As defined by the comparison between the change
in the treated group vs the change in the
control. FATS Familial Atherosclerosis
Treatment Study STARS St Thomas
Atherosclerosis Regression Study HARP Harvard
Atherosclerosis Reversibility Project LCAS
Lipoprotein and Coronary Atherosclerosis
Study. Brown BG et al. Circulation
1993871781?1791. Herd JA. Am J Med
199810442S?49S. Sacks FM et al. Lancet
19943441182?1186.
2
Patients with CHD Risk Factors ReachingNCEP
LDL-C Goals
Patients reaching target at Week 12 (starting
doses)
71
41
34
16
Atorvastatin(n86)
Fluvastatin(n85)
Lovastatin(n86)
Simvastatin(n87)
Plt0.05 vs other statins. Results from a 54-week,
open-label, randomized study in 344 patients with
risk factors for CHD.Starting doses were
atorvastatin 10 mg/day, fluvastatin 20 mg/day,
lovastatin 20 mg/day and simvastatin 10
mg/day. Hunninghake D et al. J Fam Pract
199847349356.
3
Which Lesions Progress to MI?
Stenosis prior to MI
14
gt70
50?70
18
lt50
MI patients (n)
MI patients (n)
68
Ambrose et al.
Nobuyoshi et al.
Giroud et al.
Little et al.
1988
1991
1992
1988
Falk E et al. Circulation 199592657?671.
4
Patients with CHD Risk Factors ReachingNCEP
LDL-C Goals
Atorvastatin(n86)
Simvastatin(n87)
Lovastatin(n86)
Fluvastatin(n85)
Results from a 54-week, open-label, randomized
study in 344 patients with risk factors for CHD.
Starting doses were atorvastatin 10 mg/day,
fluvastatin 20 mg/day, lovastatin 20 mg/day and
simvastatin 10 mg/day. Doses were doubled in
non-responders at 12-week intervals. Hunninghake
D et al. J Fam Pract 199847349356.
5
Committee Members
Advisory/Safety Bertram Pitt, MD,
ChairpersonUniversity of Michigan Medical
CenterAnn Arbor, Michigan, USA W. Virgil Brown,
MDAtlanta VA Hospital/Emory University School
of MedicineAtlanta, Georgia, USA David Waters,
MDHartford HospitalHartford, Connecticut, USA
Endpoint Robert DiBianco, MD, ChairpersonWashing
ton Adventist HospitalTakoma Park, Maryland,
USA Kim A. Eagle, MDThe University of Michigan
Medical CenterAnn Arbor, Michigan,
USA Christopher M. OConnor, MDDuke University
Medical CenterDurham, North Carolina,
USA Attilio Maseri, MDUniversita Cattolica del
Sacro CuoreRome, Italy
Pitt B et al. N Engl J Med 199934170?76.
6
Principal Investigators
United States (14 centers)Alan S. Brown, MD,
Lombard, ILDaniel A. Eisenberg, MD, Burbank,
CAMichael D. Ezekowitz, MD, PhD, West Haven,
CTRobert L. Feldman, MD, Ocala, FLC. Michael
Gibson, MD, West Roxbury, MAStephen W. Halpern,
MD, Santa Rosa, CAMirle A. Kellett, MD,
Portland, MELeonard Keilson, MD, Portland, ME
David Lu, MD, Washington, DCBenjamin
MacCallister, MD, Ypsilanti, MIRonald
VandenBelt, MD, Ypsilanti, MIMichael Miller, MD,
Baltimore, MDWilliam ONeill, MD, Royal Oak,
MICarl J. Pepine, MD, Gainesville, FLAnthony L.
Pucillo, MD, Valhalla, NYRobert Wilensky, MD,
Philadelphia, PA Canada (7 centers)Todd J.
Anderson, MD, Calgary, AltaRonald G. Carere, MD,
Vancouver, BCGilles Cote, MD, Montreal, QueJohn
Ducas, MD, Winnipeg, Man
Europe (16 centers)Jean-Leon Guermonprez, MD,
Paris, FranceJacques Puel, MD, Toulouse,
FranceAlex Frey, MD, Bad Krozingen,
GermanyFranz X. Kleber, MD, Berlin,
GermanyHarald Mudra, MD, München,
GermanyAndreas Zeiher, MD, Frankfurt,
GermanyPierino Zardini, MD, Verona, ItalyPim de
Feyter, PhD, MD, Rotterdam, The NetherlandsAd J.
van Boven, MD, Groningen, The NetherlandsEnric
Domingo, MD, Barcelona, SpainCarlos Macaya, MD,
Madrid, SpainWolfgang Kiowski, MD, Zurich,
SwitzerlandNicholas H. Brooks, MD, Manchester,
UK Anthony R. Rickards, MD, London, UKDavid H.
Roberts, MD, Blackpool, UK Adam D. Timmis, MD,
London, UK
Serge Lepage, MD, Sherbrooke, QueLeonard
Schwartz, MD, Toronto, OntLawrence Title, MD,
Halifax, NS
Pitt B et al. N Engl J Med 199934170?76.
7
Patients Reaching NCEP LDL-C Goals
90
79
79
55
Atorvastatin1080 mg(n166)
Simvastatin1040 mg(n164)
Lovastatin2080 mg(n167)
Fluvastatin2040 mg(n165)
Plt0.02 vs other statins. Data from a 54-week,
randomized, open-label, dose-titration trial
using a protocol based on NCEP guidelines.
Patients may also have received cholestyramine if
their treatment target was not achieved at the
maximum statin dose. Koren MJ et al.
Pharmacoeconomics 1998145970.
8
Patients Reaching NCEP LDL-C Goals
Atorvastatin(n166)
Simvastatin(n164)
Lovastatin(n167)
Fluvastatin(n165)
Data are from a 54-week, randomized, open-label,
dose-titration trial using a protocol based on
NCEP guidelines. Patients may also have received
cholestyramine if their treatment target was not
achieved at the maximum statin dose. Koren MJ et
al. Pharmacoeconomics 1998145970.
9
Study Design and Inclusion Criteria
Patients recommended for angioplasty CAD ?1
lesion ?50 stenosisLDL-C ³115 mg/dL (?3.0
mmol/L) TG 500 mg/dL (5.6 mmol/L)Bruce
protocol treadmill test or 20-W/minbicycle
exercise test ?4 min CCS angina class 2
Atorvastatin 80 mg/day usual medical
therapy (n164)
Angioplastyusual care, including lipid
lowering(n177)
18 months
- Primary efficacy parameter
- Occurrence of ischemic events (death from cardiac
causes, resuscitation after cardiac arrest,
nonfatal MI, CVA, CABG, angioplasty, worsening
angina verified by objective evidence resulting
in hospitalization) - Additional parameters
- Time to first ischemic event, change in lipid
parameters, safety
Pitt B et al. N Engl J Med 199934170?76.
10
Patients with CHD Reaching NCEP LDL-C Goal
67
53
Atorvastatin 1040 mg(n1897)
Simvastatin 1040 mg(n959)
Plt0.001 vs simvastatin. Results from a 14-week,
open-label, parallel-group, randomized,
dose-titration study. The LDL-C goal was lt100
mg/dL (2.6 mmol/L). März W et al. Am J Cardiol
199984713
11
Major Exclusion Criteria
- Left main disease or 3-vessel disease
- Unstable angina
- MI within previous 14 days
- Known ejection fraction lt40 or NYHA Class III
or IV congestive heart failure - Previous CABG, unless grafts were patent and
patient did not have 3-vessel disease
- CABG recommended basedon current angiogram
- Percutaneous revascularization in previous 6
months - Known hypersensitivity to HMG-CoA
reductaseinhibitors - AST/ALT gt2 x ULN
- CPK gt3 x ULN orunexplained elevations
Pitt B et al. N Engl J Med 199934170?76. McCormi
ck LS et al. Am J Cardiol 1997801130?1133.
12
Patients Reaching Total-C Goal lt5 mmol/L
(lt190mg/dL)
Atorvastatin (n691) Simvastatin (n337)
83
73
58
66
59
38
43
25
Week number 6 12 18 24 Atorvastatin
(mg) 10 1020 1040 1080 Simvastatin
(mg) 10 1020 1040 1040
( cholestyramine)
Plt0.005 vs simvastatin. Data are from an
open-label, randomized, 6-month, treat-to-target
study. Patients received atorvastatin (up to 80
mg) or simvastatin (up to 40 mg plus
cholestyramine if necessary). Doses were
successively titrated up to the maximum at 6-week
intervals. Mean baseline Total-C values were 7.4
mmol/L (286 mg/dL) and 7.3 mmol/L (282 mg/dL) for
atorvastatin and simvastatin respectively. OBrien
RC et al. Presented at the 71st European
Atherosclerosis Society Congress, 2629 May,
1999, Athens, Greece.
13
Overview of Study Procedures
- Treatment phase
- Patients randomized to atorvastatin discontinued
other lipid-lowering medication and immediately
began atorvastatin 80 mg/day - Patients randomized to angioplasty/usual care
(UC) underwent angioplasty followed by usual
care - ? usual care may or may not have included
lipid-lowering therapy (e.g diet, behavior
modification, or medication) - ? angioplasty may or may not have included
stenting - ? usual care was determined by investigator
or patients primary physician
Pitt B et al. N Engl J Med 199934170?76. McCormi
ck LS et al. Am J Cardiol 1997801130?1133.
14
Patients Reaching Total-C Goal at Varying
Baseline Cholesterol Levels
71
54
43
21
27
5
7
0
5.66.5 (215250)
6.67.5 (255290)
7.68.5 (295340)
gt8.5 (gt340)
Plt0.01 vs simvastatin. Data are from an
open-label, randomized, 6-month, treat-to-target
study. Treatment goal was Total-C lt5.0 mmol/L
(190 mg/dL). Data on file, Parke-Davis.
15
Primary Efficacy Assessment
- Occurrence of an ischemic event in each treatment
group - Ischemic event was defined as occurrence of at
least one of the following
? angioplasty (other than the original procedure
in angioplasty/usual care group) ? worsening
angina verified by objective evidence resulting
in hospitalization
- ? cardiac death
- ? resuscitation after cardiac arrest
- ? nonfatal MI
- ? CVA
- ? CABG
CVAcerebrovascular accident. Pitt B et al. N
Engl J Med 199934170?76.
16
Additional Efficacy Assessments
- Additional efficacy assessments included
- Time from randomization to first ischemic event
- Percent change from baseline in
- ? Total-C
- ? LDL-C
- ? HDL-C
- ? TG
Pitt B et al. N Engl J Med 199934170?76. McCormi
ck LS et al. Am J Cardiol 1997801130?1133.
17
Statistical Analysis
- Assumptions included 35 ischemic event rate in
angioplasty/UC-treated group and 20 event rate
in atorvastatin-treated group - Sample size provided 85 power with 5
significance level to detect a 15 between-group
difference in incidence of ischemic events (CMH) - Two interim analyses performed significance
level adjusted from 0.05 to 0.045 accordingly
CMH Cochran-Mantel-Haenszel. Pitt B et al. N
Engl J Med 199934170?76.
18
Baseline Patient Characteristics
Atorvastatin (n164) Angioplasty/UC
(n177) Mean age (years) 59 58 Male/Female
() 79/21 89/11 Mean ejection fraction
() 61 61 Single vessel disease
() 57 56 Double vessel disease () 43 44 Mean
stenosis 80 81 Prior MI () 45 40 Patients
with target lesion () LAD 43 30 LCX 36 36 RCA 36
36 CCS angina class () Asymptomatic 18 15 Class
I 45 40 Class II 37 44 Class III-IV 1 2
Pitt B et al. N Engl J Med 199934170?76.
19
Ischemic Events
Number () Atorvastatin Angioplasty/UC of
patients n164 n177 ? Any ischemic event 22
(13) 37 (21) ?36 Death 1 (0.6) 1 (0.6)
Resuscitated cardiac arrest 0 (0.0) 0 (0.0)
Nonfatal MI 4 (2.4) 5 (2.8) CVA 0 (0.0) 0
(0.0) CABG 2 (1.2) 9 (5.1) Angioplasty 18
(11.0) 21 (11.9) Worsening angina with
objective evidence hospitalization 11
(6.7) 25 (14.1)
P0.048 vs an adjusted significance level of
P0.045 atorvastatin vs angioplasty/UC. Pitt B et
al. N Engl J Med 199934170?76.
20
Ischemic Events
?36 difference
(P0.048)
21
13
n22 of 164
n37 of 177
P0.048 vs an adjusted significance level of
P0.045 atorvastatin vs angioplasty/UC. Pitt B et
al. N Engl J Med 199934170?76.
21
Time to First Ischemic Event
Angioplasty/UC (n177)
Atorvastatin (n164)
P0.027
Time since randomization (months)
Pitt B et al. N Engl J Med 199934170?76.
22
Change in LDL-C From Baseline
Atorvastatin (n164)
(3.9)
147 (3.8)
Angioplasty/UC (n177)
145 (3.7)
Joint European recommendations 115 mg/dL (3.0
mmol/L)
(3.2)
119 (3.1)
NCEP ATP-II guidelines 100 mg/dL (2.6 mmol/L)
(2.6)
77 (2.0)
(1.9)
(1.3)
(0.6)
Baseline End of study (18 months)
Plt0.05 vs angioplasty/UC At randomization, 26
and 19 of patients were taking lipid-lowering
medication in the atorvastatin 80 mg/day and
angioplasty/UC arms, respectively. 73 of the
angioplasty/UC patients were on lipid-lowering
therapy at some time during the study. Pitt B et
al. N Engl J Med 199934170?76.
23
Summary of Lipid Parameters
Atorvastatin baseline
(6.5)
Angioplasty/UC baseline
? 10
223 (5.8)
222 (5.7)
Atorvastatin end of study
? 10
Angioplasty/UC end of study
197 (5.1)
(5.2)
? 31
? 11
168 (1.9)
165 (1.9)
161 (1.8)
? 18
151 (3.9)
147 (3.8)
(3.9)
145 (3.7)
139 (1.6)
? 46
119 (3.1)
(2.6)
77 (2.0)
? 8
? 11
47 (1.22)
45 (1.16)
46 (1.2)
(1.3)
43 (1.1)
Plt0.05 vs angioplasty/UC Baseline values
represented patients at randomization without a
washout period from existing lipid-lowering
therapy. Note 73 of angioplasty/UC-treated
patients were on lipid-lowering medication at
some time during the study. Pitt B et al. N Engl
J Med 199934170?76.
24
Incidence of First Ischemic Event by Time
Angioplasty/UC
Atorvastatin
24 difference
46 difference
11
10
7
6
Pitt B et al. N Engl J Med 19993417076.
25
Safety Evaluation
- Elevations in AST or ALT (consecutive elevations
gt3 x ULN) - ? 4 (2.4) atorvastatin-treated patients
- ? none in angioplasty/UC-treated patients
- Elevations in CPK (gt10 x ULN)
- ? none in either treatment group
- There were no clinically significant differences
in adverse event rates between the two treatment
groups - ? in this study, eight patients discontinued
atorvastatin treatment due to an adverse event,
but remained in the study
Pitt B et al. N Engl J Med 199934170?76.
26
Conclusions
- Over the 18-month study period, aggressive lipid
lowering with atorvastatin in stable CAD
patients - ? resulted in a mean LDL-C level of 77 mg/dL
and cardiovascular benefit was achieved - ? produced a 36 reduction in total ischemic
events (P0.048) - ? significantly delayed the time to first
ischemic event - ? was well-tolerated
Although this difference did not reach the level
of significance as adjusted for interim analyses
(P0.045), it did reach the conventional 5 level
of significance. Pitt B et al. N Engl J Med
199934170?76.