Electrolytic Cells: - PowerPoint PPT Presentation
1 / 5
Title:
Electrolytic Cells:
Description:
... effect' at the top of the SRP table are more easily oxidized than water. ... (Br- and Cl- always oxidize before H2O due to overpotential effect) ... – PowerPoint PPT presentation
Number of Views:499
Avg rating:3.0/5.0
Title: Electrolytic Cells:
1
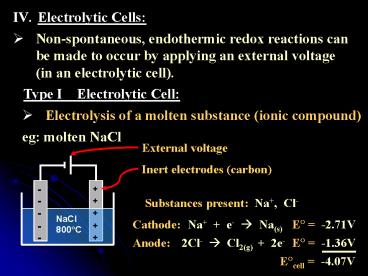
- Electrolytic Cells
- Non-spontaneous, endothermic redox reactions can
be made to occur by applying an external voltage
(in an electrolytic cell).
Type I Electrolytic Cell
- Electrolysis of a molten substance (ionic
compound)
eg molten NaCl
External voltage
Inert electrodes (carbon)
Substances present Na, Cl-
Cathode
Na e- ? Na(s)
E? -2.71V
Anode
2Cl- ? Cl2(g) 2e-
E? -1.36V
E?cell -4.07V
2
- At least 4.07V are required to force reaction to
occur. (In practice more than this is required)
- This is how Na(s) and Cl2(g) are produced
industrially.
Type II Electrolytic Cell
- Electrolysis of an aqueous solution of an ionic
compound.
eg NaI
Inert electrodes (platnum or carbon)
Substances present Na, I- , H2O
- Must determine which reactions take place.
3
- Two choices for reduction
a)
Na e- ? Na
E? -2.71V
b)
2H2O 2e- ? H2 2OH-
E? -0.41V
b) will occur because it requires the least
voltage.
- Two choices for oxidation
a)
2I- ? I2 2e-
E? -0.53V
b)
H2O ? ½O2 2H 2e-
E? -0.82V
a) will occur because it requires the least
voltage.
Anode
2I- ? I2 2e-
E?cell -0.94V
Cathode
2H2O 2e- ? H2 2OH-
- This electrolytic cell requires at least 0.94V
- The products are H2(g) I2(s). (also NaOH)
4
Overpotential effect
- In practice, the actual voltage required to power
an electrolytic cell is always greater than the
calculated voltage.
- The difference between the calculated voltage and
the voltage actually required is called the
overpotential.
- This overpotential is higher for reactions
involving the oxidation or reduction of water.
- Compounds included in the overpotential effect
at the top of the SRP table are more easily
oxidized than water.
- Compounds included in the overpotential effect
at the bottom of the SRP table are more easily
reduced than water.
(Br- and Cl- always oxidize before H2O due to
overpotential effect)
5
Practice
- Predict anode and cathode half reactions during
electrolysis of these 1.0M solutions.
- KI
- HCl
- NaF
- NaBr
- HF
- Calculate the minimum amount of voltage required
to power each of the above. (E?cell)
- Draw a labeled diagram of 1a).